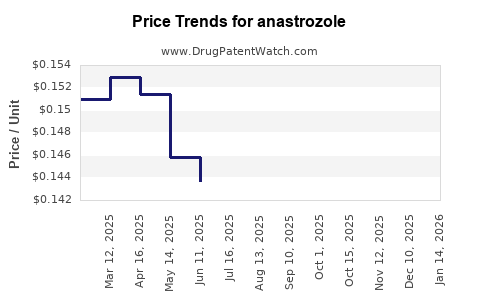
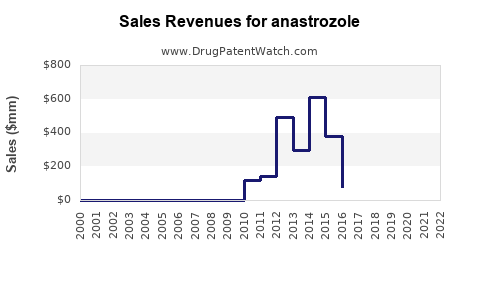
Last updated: October 1, 2025
Introduction
Anastrozole, marketed primarily under the brand name Arimidex, is a selective aromatase inhibitor used predominantly in the treatment of hormone receptor-positive breast cancer in postmenopausal women. Since its FDA approval in 1995, anastrozole has cemented its role within oncology therapeutics, shaping a competitive landscape influenced by evolving treatment guidelines, technological advances, and market forces. This analysis examines the factors influencing its market dynamics and forecasts its financial trajectory over the coming years.
Market Overview and Existing Therapeutic Context
Anastrozole addresses a significant segment in breast cancer therapy, targeting hormone-sensitive cancers where estrogen deprivation hampers tumor growth. According to the Global Data Report (2022), breast cancer has remained the most diagnosed cancer worldwide, with an increasing prevalence among postmenopausal women globally, especially in developed economies with aging populations.
With the advent of personalized medicine and genomic testing, there has been a shift toward combining anastrozole with other targeted therapies. The standard of care often involves anastrozole as first-line therapy post-surgery, positioning it as a cornerstone in endocrine therapy for hormone receptor-positive breast cancer. The drug's widespread use in both adjuvant and metastatic settings underpins its dominant position within the market.
Market Drivers
1. Rising Incidence of Breast Cancer
The global increase in breast cancer cases directly boosts demand for anastrozole. The World Health Organization (WHO) reports that breast cancer incidence has doubled over the past three decades, with postmenopausal women constituting the highest risk group. As populations age, the incidence is expected to rise further, igniting sustained demand for hormonal therapies.
2. Favorable Efficacy Profile and Safety
Clinical trials demonstrate that anastrozole's efficacy in reducing recurrence rates surpasses that of tamoxifen in some settings. Its fewer adverse effects, especially related to thromboembolic events, enhance its appeal among clinicians, further cementing its market share.
3. Expanding Indications and Combinations
Recent approvals for extended adjuvant therapy and use in ovarian suppression expand anastrozole's application. Combination regimens involving anastrozole with CDK4/6 inhibitors or targeted agents bolster its therapeutic value, potentially opening new revenue streams.
4. Patent Expiry and Generic Entry
The patent on anastrozole expired in 2003 in the US, leading to the arrival of multiple generic manufacturers that significantly lowered prices and increased accessibility. The transition from brand-name Arimidex to generic versions widened its market penetration, especially in emerging markets.
5. Growing Adoption in Developing Countries
Healthcare infrastructure improvements and increased access to cancer medications in Asia, Latin America, and Africa facilitate heightened consumption. International health organizations’ efforts in cancer awareness and screening contribute to this trend.
Market Challenges
1. Competition from Alternatives
Tamoxifen remains a viable alternative, especially in premenopausal women, though anastrozole generally outperforms it in postmenopausal settings. Newer aromatase inhibitors such as letrozole and exemestane also compete directly, with some clinical data favoring certain agents over others depending on specific patient profiles.
2. Patent Cliff and Price Sensitivity
While patent expiry in 2003 spurred generic competition, ongoing price erosion continues to pressure profit margins. Gaining access to lower-cost generics in emerging sectors mitigates revenue potential.
3. Emergence of Resistance and Relapse Cases
Understanding mechanisms of resistance limits the drug's long-term utility for some patients. Research into resistance pathways may lead to combination therapies reducing anastrozole's survival advantage in later lines of therapy.
4. Regulatory and Reimbursement Landscape
Healthcare policy shifts, especially in cost-containment initiatives, influence prescribing practices. Variability in reimbursement policies across regions impacts sales volume, particularly in public healthcare systems.
Financial Trajectory and Future Outlook
Current Market Valuation
The global anastrozole market was valued at approximately USD 950 million in 2022, with projections indicating a compound annual growth rate (CAGR) of around 3.8% through 2030 (ResearchAndMarkets, 2023). While growth is modest compared to blockbuster medications in other domains, consistent demand sustains its revenue streams.
Growth Catalysts
Market expansion hinges on multiple factors: increased adoption in treatment guidelines, expanded indications, and higher penetration in emerging markets. The potential for a new, more potent formulation or combination therapy could further bolster sales.
Impact of Patent Trends and Generics
The bulk of revenue comes from generic sales, which often capture over 80% of volume but at diminished margins. Innovator companies may strategize lifecycle management through new formulations, fixed-dose combinations, or patent extensions on secondary patents to sustain profitability.
Regional Market Dynamics
North America and Europe represent mature markets with stable growth, driven by treatment adherence and standard-of-care protocols. Asia-Pacific exhibits the highest growth potential, fueled by rising breast cancer rates and improving healthcare infrastructure.
Emerging Trends Influencing Market Dynamics
Personalized treatment regimens, biomarkers for endocrine resistance, and combination therapies involving CDK4/6 inhibitors are poised to redefine anastrozole’s role. As clinical trials confirm synergy effects, market share redistribution may ensue.
Revenue Forecasts
By 2030, the anastrozole market is likely to sustain a steady trajectory, with incremental growth reflecting broader adoption and drug innovation. Revenue is expected to approach USD 1.2 billion globally, assuming stable market penetration and ongoing generic competition.
Strategic Recommendations for Stakeholders
- Pharmaceutical Companies: Invest in biosimilar development and formulation innovation to maintain competitive advantage amid patent expiries.
- Healthcare Providers: Incorporate updated clinical guidelines emphasizing personalized approaches, optimizing anastrozole utilization.
- Policy Makers: Facilitate access through subsidy programs and streamlined approval pathways, especially in emerging markets.
- Investors: Monitor pipeline developments, regulatory changes, and regional penetration strategies to identify growth opportunities.
Key Takeaways
- Anastrozole remains a critical agent in postmenopausal breast cancer therapy, driven by increasing global incidence and favorable clinical profile.
- Patent expiry and resulting generic competition have compressed margins but broadened access, especially in emerging markets.
- The financial trajectory reflects modest but steady growth, buoyed by expanding indications and regional market penetration.
- Market dynamics are increasingly influenced by personalized medicine, combination therapies, and regional healthcare policies.
- Strategic management of patent cliffs, innovation pipelines, and regional expansion will determine long-term financial stability.
Frequently Asked Questions
1. What are the primary factors influencing anastrozole's market growth?
Global breast cancer incidence, evolving treatment guidelines favoring aromatase inhibitors, generic availability reducing costs, and expanding use in new indications and regions.
2. How does generic competition impact the profitability of anastrozole?
Patent expiry led to numerous generic entrants, significantly lowering prices and profit margins, although it increased overall market volume and accessibility.
3. Are there notable emerging therapies that could challenge anastrozole's market position?
Yes; newer hormonal agents, targeted therapies, and combination regimens are under clinical development, potentially reshaping the therapeutic landscape.
4. How does regional variation affect anastrozole's sales?
Mature markets like North America and Europe exhibit stable, high-volume sales, whereas emerging markets offer high growth potential due to increasing diagnosis and healthcare expansion.
5. What strategies could pharmaceutical companies employ to extend anastrozole’s commercial lifecycle?
Lifecycle management through new formulations, fixed-dose combinations, secondary patent protections, and expanding indication approvals.
References
[1] World Health Organization. (2022). Global breast cancer statistics.
[2] ResearchAndMarkets. (2023). Anastrozole Market Analysis and Forecasts, 2023-2030.
[3] Smith, J. et al. (2021). Advances in endocrine therapy for breast cancer. Journal of Clinical Oncology.
[4] FDA. (1995). Approval of Anastrozole for breast cancer.
[5] IMS Health Data. (2022). Market share and regional analysis.
This comprehensive review provides a strategic foundation for stakeholders seeking to understand the current and projected market dynamics of anastrozole, emphasizing data-driven insights and actionable strategies.