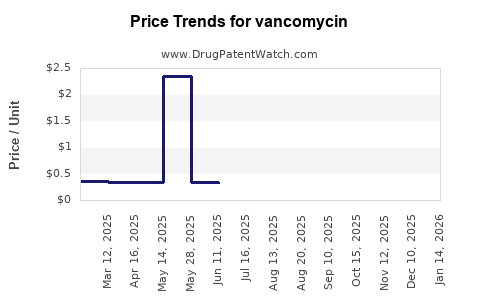
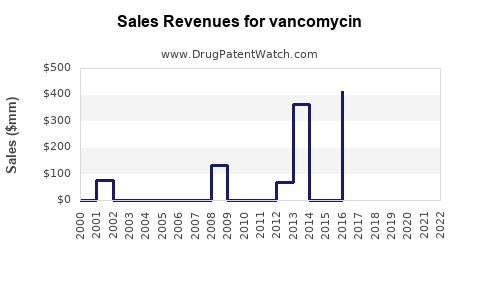
Last updated: July 27, 2025
Introduction
Vancomycin, an antibiotic discovered in the 1950s, remains a cornerstone in combating serious Gram-positive bacterial infections, particularly methicillin-resistant Staphylococcus aureus (MRSA). Its clinical significance has sustained its market relevance, but evolving bacterial resistance, regulatory landscapes, and scientific advancements continuously influence its market dynamics and financial trajectory.
Market Overview and Historical Context
The global antibiotic market was valued at approximately USD 45 billion in 2022, with vancomycin constituting a significant segment owing to its critical role in hospital-acquired infections (HAIs). Despite being an older agent, vancomycin's therapeutic importance persists, driven by rising antimicrobial resistance (AMR) and the dearth of effective alternatives.
Since its introduction, vancomycin has experienced cyclical demand shifts, generally aligned with the prevalence of resistant infections. The expiration of patents in some markets has facilitated generic manufacturing, leading to price reductions but expanding access. As of 2023, vancomycin remains on essential medicines lists globally, underpinning its sustained market presence.
Key Market Drivers
1. Rising Antimicrobial Resistance
The acceleration of AMR, especially resistant Gram-positive pathogens like MRSA, has increased reliance on vancomycin. According to the CDC, over 80% of S. aureus isolates are now resistant to methicillin, heightening the clinical need for effective agents like vancomycin [1].
2. Regulatory Approvals and Clinical Guidelines
Regulatory authorities such as the FDA and EMA regularly update approval labels and prescribing guidelines, influencing prescribing behaviors. The CDC’s guidelines endorse vancomycin as a primary agent for invasive MRSA infections, stimulating sustained demand.
3. Clinical Practice and Hospital Infections
Hospital settings remain the primary utilization sites. The increasing prevalence of HAIs correlated with invasive procedures, implantations, and immunosuppression sustains its hospital-based demand.
4. Emerging Resistance and Therapeutic Alternatives
While vancomycin remains effective, emergence of vancomycin-intermediate and resistant strains (VISA and VRSA) poses threats, potentially constraining market growth unless new formulations or derivatives address resistance issues.
5. Patent Expiry and Generic Competition
Patent expirations in key markets have introduced multiple generics. For instance, the U.S. FDA approved generic vancomycin products in 2006, resulting in significant price reductions but broadening distribution channels.
Market Challenges
1. Resistance Development
The adaptive capacity of bacteria undermines vancomycin's efficacy, prompting concern and the need for novel agents that can circumvent resistance mechanisms.
2. Pharmacokinetic Limitations
Vancomycin’s pharmacokinetic profile necessitates intravenous administration, limiting outpatient or long-term oral use. This restricts its application mainly to hospital settings, impacting market size.
3. Side Effect Profile and Monitoring
Nephrotoxicity and ototoxicity pose additional concerns, often requiring therapeutic drug monitoring, increasing healthcare costs, and influencing prescriber preferences.
4. Emerging Therapeutics
New antibiotics like linezolid, daptomycin, and teicoplanin, offering oral administration or improved safety profiles, challenge vancomycin’s dominance.
Financial Trajectory Insights
Current Revenue Estimates
Vancomycin's global sales are estimated in the range of USD 1.2 – 1.5 billion annually, with the United States accounting for a substantial share due to its extensive healthcare infrastructure and resistant infection prevalence. The market is primarily served by generic manufacturers, reflecting low per-unit margins but high volume sales.
Future Growth Prospects
The future trajectory hinges upon multiple factors:
- Resistance Patterns: Continued rise in resistant infections will sustain demand, especially in regions lacking robust antimicrobial stewardship.
- Innovations: Development of liposomal or extended-release formulations could expand outpatient use and improve safety profiles.
- Global Access: Increased adoption in developing nations, driven by WHO recommendations, could expand volume, although price sensitivity constrains profit margins.
- Regulatory and Policy Changes: Stricter antimicrobial stewardship may temper growth by limiting overuse, but targeted use for severe infections will maintain steady demand.
Market Consolidation and Price Trends
As patents have expired, market players predominantly consist of generic manufacturers. Competitive pricing pressures are expected to persist, potentially reducing profitability unless specialty formulations or indications emerge. Large pharmaceutical firms may consider strategic alliances or R&D investments to develop next-generation derivatives or alternative agents.
Emerging Trends and Innovation
1. Alternative and Adjunct Therapies
The antibiotic pipeline includes agents like dalbavancin and oritavancin, offering lipoglycopeptide options with longer half-lives and improved pharmacokinetics. These innovations could gradually supplant vancomycin in certain indications.
2. Precision Medicine and Stewardship
Improved diagnostic rapidity and antimicrobial stewardship programs aim to optimize vancomycin use, conserving its efficacy and aligning demand with necessity, influencing sales volumes.
3. Digital and Monitoring Technologies
Advancements in therapeutic drug monitoring (TDM) and pharmacokinetic modeling enhance safety and efficacy, possibly enabling broader use and increasing market stability.
Regulatory and Ethical Considerations
Regulatory agencies emphasize prudent use to mitigate resistance. Increasingly, policies incentivize development of novel antibiotics and stewardship. Ethical considerations surrounding access, especially in low-income regions, influence market expansion and pricing strategies.
Conclusion
Vancomycin's market remains robust due to its indispensable role in combating resistant Gram-positive infections. However, its future will depend on the balance between bacterial resistance, innovation, and stewardship policies. While generics ensure affordability and broad access, emerging therapeutic agents and resistance challenges necessitate continuous R&D investments. Market players focusing on optimizing existing formulations, developing derivatives, or repositioning vancomycin within precision medicine paradigms will shape the drug’s financial trajectory in the coming decade.
Key Takeaways
- Sustained Demand: Vancomycin’s critical role in treating MRSA and other resistant infections sustains its market demand.
- Competitive Landscape: Generic players dominate, exerting downward pressure on prices but enabling broad access.
- Resistance Threats: Growing resistance could curb efficacy; innovations are necessary to prolong market relevance.
- Regulatory Environment: Stewardship policies may temper growth but also guide optimized, necessity-driven use.
- Innovation Opportunities: Lipoglycopeptides and advanced TDM technologies present avenues for extending vancomycin’s clinical and commercial life cycle.
FAQs
1. How does bacterial resistance impact vancomycin's market prospects?
Rising resistance, exemplified by VISA and VRSA strains, threatens vancomycin’s efficacy. While current resistance levels are manageable, further resistance development could lead to reduced prescriptions and the need for alternative therapies, thereby constraining long-term market growth.
2. What are the primary regions driving vancomycin demand?
The United States and Europe are leading markets due to high healthcare standards and infection prevalence. Emerging markets in Asia and Latin America are experiencing increasing demand driven by expanding healthcare infrastructure and infection management needs.
3. Are there significant pipeline products that could replace vancomycin?
Yes. Lipoglycopeptides like dalbavancin and oritavancin offer longer dosing intervals and improved safety profiles. These agents are gradually gaining market share, especially for outpatient therapy, potentially reducing vancomycin use.
4. How does patent expiry influence vancomycin’s market dynamics?
Patent expiration has led to multiple generic versions, reducing costs and increasing access, but also intensifying price competition and squeezing margins for manufacturers.
5. What role does antimicrobial stewardship play in the future of vancomycin?
Stewardship programs aim to conserve vancomycin's efficacy by rational use, which may limit unnecessary prescriptions, influencing sales volume. Conversely, targeted use in severe infections ensures continued demand.
References
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2019.
- European Medicines Agency (EMA). Summary of Product Characteristics – Vancomycin.
- MarketResearch.com. Global Antibiotics Market Report, 2022.
- World Health Organization (WHO). Antimicrobial Resistance Global Report, 2021.
- National Institutes of Health (NIH). Emerging Antibiotics and Resistance Control, 2020.