Sirolimus - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for sirolimus and what is the scope of freedom to operate?
Sirolimus
is the generic ingredient in four branded drugs marketed by Nobelpharma, Aadi, Pf Prism Cv, Amneal, Apotex, Hetero Labs Ltd V, MSN, Novitium Pharma, Torrent, Alkem Labs Ltd, Dr Reddys, Glenmark Pharms Ltd, and Zydus Pharms, and is included in fourteen NDAs. There are six patents protecting this compound and two Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Sirolimus has one hundred and fifty patent family members in thirty-two countries.
There are twenty-one drug master file entries for sirolimus. Nineteen suppliers are listed for this compound.
Summary for sirolimus
| International Patents: | 150 |
| US Patents: | 6 |
| Tradenames: | 4 |
| Applicants: | 13 |
| NDAs: | 14 |
| Drug Master File Entries: | 21 |
| Finished Product Suppliers / Packagers: | 19 |
| Raw Ingredient (Bulk) Api Vendors: | 68 |
| Clinical Trials: | 687 |
| Patent Applications: | 4,252 |
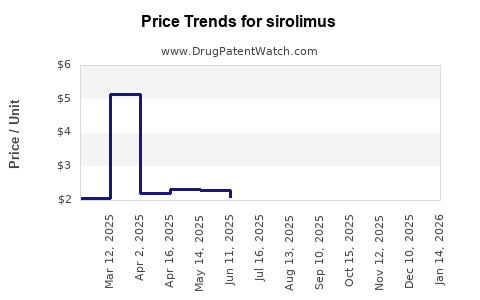
| Drug Prices: | Drug price trends for sirolimus |
| What excipients (inactive ingredients) are in sirolimus? | sirolimus excipients list |
| DailyMed Link: | sirolimus at DailyMed |
Recent Clinical Trials for sirolimus
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Case Comprehensive Cancer Center | Phase 2 |
| Stanford University | Phase 1/Phase 2 |
| Aadi Bioscience, Inc. | Phase 2 |
Pharmacology for sirolimus
| Drug Class | Kinase Inhibitor mTOR Inhibitor Immunosuppressant |
| Mechanism of Action | Protein Kinase Inhibitors mTOR Inhibitors |
| Physiological Effect | Decreased Immunologic Activity |
Medical Subject Heading (MeSH) Categories for sirolimus
Anatomical Therapeutic Chemical (ATC) Classes for sirolimus
US Patents and Regulatory Information for sirolimus
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pf Prism Cv | RAPAMUNE | sirolimus | TABLET;ORAL | 021110-001 | Aug 25, 2000 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Glenmark Pharms Ltd | SIROLIMUS | sirolimus | TABLET;ORAL | 208691-002 | Oct 16, 2020 | AB | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Aadi | FYARRO | sirolimus | POWDER;INTRAVENOUS | 213312-001 | Nov 22, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for sirolimus
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pf Prism Cv | RAPAMUNE | sirolimus | TABLET;ORAL | 021110-002 | Aug 22, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | RAPAMUNE | sirolimus | TABLET;ORAL | 021110-002 | Aug 22, 2002 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | RAPAMUNE | sirolimus | TABLET;ORAL | 021110-004 | Jan 25, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for sirolimus
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Pfizer Europe MA EEIG | Rapamune | sirolimus | EMEA/H/C/000273 Rapamune is indicated for the prophylaxis of organ rejection in adult patients at low to moderate immunological risk receiving a renal transplant. It is recommended that Rapamune be used initially in combination with ciclosporin microemulsion and corticosteroids for 2 to 3 months. Rapamune may be continued as maintenance therapy with corticosteroids only if ciclosporin microemulsion can be progressively discontinued., , Rapamune is indicated for the treatment of patients with sporadic lymphangioleiomyomatosis with moderate lung disease or declining lung function., |
Authorised | no | no | no | 2001-03-13 | |
| Plusultra pharma GmbH | Hyftor | sirolimus | EMEA/H/C/005896 Hyftor is indicated for the treatment of facial angiofibroma associated with tuberous sclerosis complex in adults and paediatric patients aged 6 years and older. |
Authorised | no | no | yes | 2023-05-15 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for sirolimus
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2017061537 | プリオンを含まないナノ粒子組成物および方法 (PRION-FREE NANOPARTICLE COMPOSITIONS AND METHODS) | ⤷ Sign Up |
| Japan | 2016011309 | 抗癌剤としてラパマイシンおよびアルブミンを含むナノ粒子 (NANOPARTICLE COMPRISING RAPAMYCIN AND ALBUMIN AS ANTICANCER AGENT) | ⤷ Sign Up |
| Eurasian Patent Organization | 202092187 | СПОСОБЫ ЛЕЧЕНИЯ ЭПИТЕЛИОИДНО-КЛЕТОЧНЫХ ОПУХОЛЕЙ | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for sirolimus
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0648494 | SPC/GB01/037 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: SIROLIMUS; REGISTERED: CH IKS 55243 20000926; UK EU/1/01/171/001-005 20010313 |
| 0763039 | C300348 | Netherlands | ⤷ Sign Up | PRODUCT NAME: TEMSIROLIMUS, DESGEWENST IN DE VORM VAN EEN; REGISTRATION NO/DATE: EU/1/07/424/001 20071119 |
| 0648494 | 24/2001 | Austria | ⤷ Sign Up | PRODUCT NAME: SIROLIMUS; NAT. REGISTRATION NO/DATE: EU/1/01/171/001 - EU/1/01/171/005 20010313; FIRST REGISTRATION: LI 5524301 20000926 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.