Penicillin v - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for penicillin v and what is the scope of freedom to operate?
Penicillin v
is the generic ingredient in fifteen branded drugs marketed by Lilly, Glaxosmithkline, Apothecon, Lederle, Wyeth Ayerst, Parke Davis, Belcher Pharms, Chartwell Rx, Mylan, Purepac Pharm, Teva, Pfizer, Bristol, Aurobindo Pharma, Hikma Pharms, Ivax Sub Teva Pharms, Sandoz, and Pharmacia And Upjohn, and is included in thirty-seven NDAs. Additional information is available in the individual branded drug profile pages.There are seventeen drug master file entries for penicillin v.
Summary for penicillin v
| US Patents: | 0 |
| Tradenames: | 15 |
| Applicants: | 18 |
| NDAs: | 37 |
| Drug Master File Entries: | 17 |
| Raw Ingredient (Bulk) Api Vendors: | 45 |
| Clinical Trials: | 190 |
| Patent Applications: | 6,406 |
| Formulation / Manufacturing: | see details |
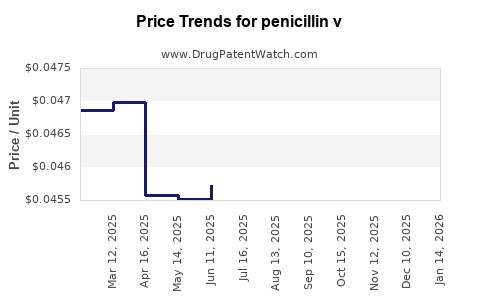
| Drug Prices: | Drug price trends for penicillin v |
| DailyMed Link: | penicillin v at DailyMed |
Recent Clinical Trials for penicillin v
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Missouri-Columbia | Phase 4 |
| Frieder Schaumburg | Phase 4 |
| Abdul El-Rabbany | Phase 4 |
Medical Subject Heading (MeSH) Categories for penicillin v
US Patents and Regulatory Information for penicillin v
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacia And Upjohn | UTICILLIN VK | penicillin v potassium | TABLET;ORAL | 061651-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Hikma Pharms | PENICILLIN V POTASSIUM | penicillin v potassium | TABLET;ORAL | 090549-001 | Oct 11, 2013 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Apothecon | VEETIDS | penicillin v potassium | TABLET;ORAL | 061411-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Apothecon | VEETIDS '125' | penicillin v potassium | FOR SOLUTION;ORAL | 061206-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Hikma Pharms | PENICILLIN V POTASSIUM | penicillin v potassium | TABLET;ORAL | 090549-002 | Oct 11, 2013 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |