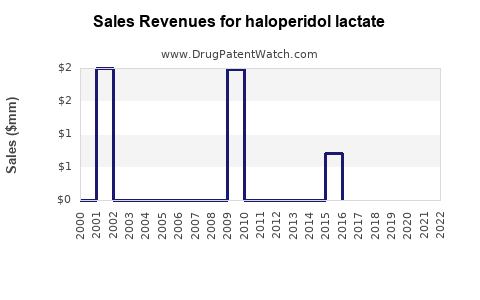
Haloperidol lactate - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for haloperidol lactate and what is the scope of freedom to operate?
Haloperidol lactate
is the generic ingredient in four branded drugs marketed by Ortho Mcneil, Alpharma, Lannett Co Inc, Morton Grove, Pharm Assoc, Rubicon, SCS, Teva, Teva Pharms, Hikma, Janssen Pharms, Abraxis Pharm, Baxter Hlthcare Corp, Epic Pharma Llc, Fosun Pharma, Fresenius Kabi Usa, Gland Pharma Ltd, Marsam Pharms Llc, Mylan Labs Ltd, Sagent Pharms, Smith And Nephew, Solopak, Teva Pharms Usa, Watson Labs, and Actavis Mid Atlantic, and is included in thirty-four NDAs. Additional information is available in the individual branded drug profile pages.Fourteen suppliers are listed for this compound.
Summary for haloperidol lactate
| US Patents: | 0 |
| Tradenames: | 4 |
| Applicants: | 25 |
| NDAs: | 34 |
| Finished Product Suppliers / Packagers: | 14 |
| Raw Ingredient (Bulk) Api Vendors: | 7 |
| Clinical Trials: | 5 |
| Patent Applications: | 297 |
| Formulation / Manufacturing: | see details |
| What excipients (inactive ingredients) are in haloperidol lactate? | haloperidol lactate excipients list |
| DailyMed Link: | haloperidol lactate at DailyMed |
Recent Clinical Trials for haloperidol lactate
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Christian Hassager | Phase 3 |
| Services Institute of Medical Sciences, Pakistan | Phase 1 |
| University Hospital, Basel, Switzerland | Phase 4 |
Pharmacology for haloperidol lactate
| Drug Class | Typical Antipsychotic |
US Patents and Regulatory Information for haloperidol lactate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solopak | HALOPERIDOL | haloperidol lactate | INJECTABLE;INJECTION | 070801-001 | Dec 14, 1987 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Ortho Mcneil | HALDOL | haloperidol lactate | CONCENTRATE;ORAL | 015922-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abraxis Pharm | HALOPERIDOL | haloperidol lactate | INJECTABLE;INJECTION | 071187-001 | Jan 20, 1987 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for haloperidol lactate
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ortho Mcneil | HALDOL | haloperidol lactate | CONCENTRATE;ORAL | 015922-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen Pharms | HALDOL | haloperidol lactate | INJECTABLE;INJECTION | 015923-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.