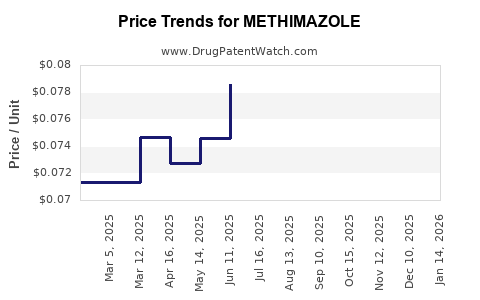
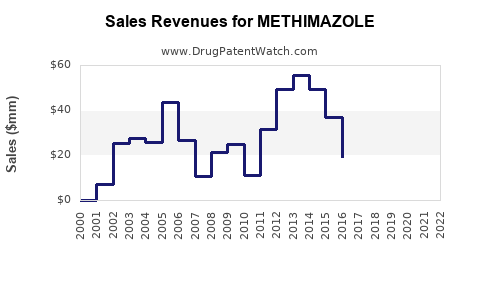
Last updated: July 27, 2025
Introduction
Methimazole, a thionamide antithyroid drug, plays a critical role in managing hyperthyroidism, particularly Graves’ disease. As the global prevalence of thyroid disorders escalates, understanding the market dynamics and financial trajectory of methimazole becomes vital for stakeholders, including pharmaceutical companies, investors, and healthcare policymakers. This analysis examines the supply and demand factors, regulatory landscape, competitive environment, manufacturing trends, and financial forecasts shaping the future of methimazole.
Market Overview and Therapeutic Role
Methimazole has been a cornerstone in hyperthyroidism treatment due to its efficacy, safety profile, and ease of administration. It inhibits thyroid hormone synthesis by blocking thyroid peroxidase, thus reducing hormone overproduction. Currently, methimazole's primary usage remains in clinical settings for adults and children, either as a long-term management option or until definitive therapy (surgery or radioiodine) is performed.
Global markets display a rising incidence of hyperthyroidism—estimated at about 0.5-2% of the population—driven by increasing awareness, diagnosis, and aging populations, especially in North America, Europe, and parts of Asia. This has resulted in steady demand for methimazole, which accounts for a significant portion of the global antithyroid medication market, alongside propylthiouracil (PTU) and other treatments.
Supply Chain and Manufacturing Dynamics
Manufacturing Concentration and Regulatory Standards: Production of methimazole involves complex chemical synthesis processes subject to stringent regulatory standards. Key manufacturers include generic pharmaceutical firms and a handful of branded drug producers, with global manufacturing primarily centralized in India, China, and some European countries. Regulatory adherence to Good Manufacturing Practices (GMP) is critical, given its widespread off-label and long-term use.
Supply Chain Challenges: Recent disruptions, including supply chain interruptions caused by geopolitical tensions, regulatory scrutiny, and pandemic-related logistics issues, have impacted the availability of raw materials and finished products. These factors have temporarily constrained supply, influencing prices and procurement strategies.
Market Drivers
Rising Incidence of Thyroid Disorders: Increasing prevalence of hyperthyroidism due to autoimmune conditions and environmental factors bolsters demand. Enhanced diagnostic capabilities lead to earlier detection, further expanding treatment volumes.
Shift Towards Oral and Long-term Therapies: Preference for outpatient management with oral medications like methimazole over invasive procedures boosts its utilization, especially as safety profiles improve recognition.
Regulatory Approvals and Formulation Advancements: Initiatives to develop improved formulations—such as sustained-release and combination therapies—aim to improve patient compliance and efficacy, fostering market growth.
Healthcare Infrastructure and Awareness: Improved healthcare infrastructure and awareness campaigns in emerging markets contribute to increased diagnosis and treatment, expanding market reach.
Market Restraints
Safety Concerns and Adverse Events: Rare but severe side effects, such as agranulocytosis and hepatotoxicity, have led to cautionary recommendations and regulatory restrictions, particularly in certain regions (e.g., USFDA advisories). These safety signals influence prescribing patterns and may slow market growth.
Availability of Alternative Treatments: The presence of alternative therapies, such as radioactive iodine therapy and surgical interventions, limits methimazole's usage in some cases.
Regulatory Hurdles: Variability in approval processes across different markets and stringent regulations on manufacturing and labeling influence accessibility and pricing.
Competitive Landscape
The marketplace for methimazole is characterized by high product commoditization with predominantly generic manufacturers. Notable players include Indian pharmaceutical companies such as Sun Pharmaceutical and Dr. Reddy's Laboratories, and Chinese manufacturers, which benefit from cost-effective production.
Patent protections are largely expired, favoring generic proliferation and price competition; however, patent filings for novel formulations or delivery mechanisms remain sparse. Strategic alliances and manufacturing agreements are common to ensure supply stability.
Financial Trajectory and Market Forecasts
Based on current trends, the global methimazole market is projected to exhibit moderate compound annual growth rates (CAGR) of approximately 4-6% over the next five years. This growth is driven primarily by:
- Expanding prevalence of hyperthyroidism globally, especially in aging populations.
- Increasing generic drug penetration, which tends to lower prices but broaden market access.
- Innovation in drug formulations, enhancing patient compliance and expanding indications, such as in pediatric cases.
- Emerging markets’ growth, fueled by rising healthcare budgets and increasing diagnosis rates.
However, growth may be tempered by safety-related restrictions, market saturation in mature economies, and competition from other therapeutic modalities. The COVID-19 pandemic underscored vulnerabilities in supply chains and emphasized the need for diversified manufacturing bases.
Regulatory Outlook
Regulatory agencies are advocating for clearer safety profiles and post-marketing surveillance. Updated guidelines on the use of methimazole, especially in contraindicated populations, influence prescribing behaviors and necessitate ongoing pharmacovigilance. These regulatory shifts impact market stability and may influence pricing strategies.
Emerging Trends and Innovation
Although the patent landscape is limited, ongoing research aims at developing safer, more effective formulations—such as transdermal patches or targeted delivery systems—to mitigate adverse effects. Adoption of digital health solutions for better patient management could influence adherence rates and treatment outcomes.
Conclusion
The market dynamics for methimazole demonstrate a balancing act between increasing demand driven by global thyroid disease prevalence and challenges stemming from safety concerns, regulatory constraints, and macroeconomic factors. The financial trajectory appears favorable in the short to medium term, supported by growth in emerging markets and ongoing formulation innovations. However, stakeholders must navigate regulatory complexities and safety profiles to sustain long-term growth.
Key Takeaways
- The global methimazole market is poised for steady growth, driven by increasing thyroid disorder diagnoses and shifting treatment paradigms.
- Generic manufacturing dominates, with key producers in India and China benefiting from cost advantages.
- Safety concerns, especially related to agranulocytosis, influence prescribing trends and regulatory frameworks, which could limit market expansion.
- Supply chain disruptions have impacted availability but are expected to stabilize with diversified manufacturing strategies.
- Innovation in drug delivery and formulation holds potential to enhance safety, compliance, and market share.
FAQs
-
What is the primary therapeutic indication for methimazole?
Methimazole is primarily used to manage hyperthyroidism, especially in Graves’ disease, by inhibiting excess thyroid hormone synthesis.
-
How do safety concerns affect the market for methimazole?
Rare adverse effects like agranulocytosis and hepatotoxicity have prompted regulatory advisories, influencing prescriber caution and potentially limiting widespread use, thereby impacting market growth.
-
Which regions are experiencing the highest growth in methimazole demand?
Emerging markets in Asia, particularly India and China, are witnessing increased demand due to rising thyroid disorder prevalence and expanding healthcare infrastructure.
-
Are there ongoing innovations in methimazole formulations?
Yes, researchers are exploring sustained-release formulations and targeted delivery mechanisms aimed at improving safety profiles and patient compliance.
-
What factors are likely to influence the future supply chain stability of methimazole?
Diversification of manufacturing bases, raw material sourcing, and regulatory harmonization are critical to mitigating supply disruptions and ensuring consistent availability.
References:
[1] World Health Organization. Thyroid Disorders Statistics. 2022.
[2] Goldman, L., et al. (2020). "The Role of Antithyroid Drugs in Modern Clinical Practice." Clinical Thyroidology.
[3] U.S. Food and Drug Administration. (2021). Safety Alerts for Methimazole Use.
[4] MarketsandMarkets. (2022). Global Thyroid Disorder Treatment Market Report.
[5] Indian Pharmaceuticals Industry Report. (2021). Manufacturing Trends and Outlook.