HYDROGEN PEROXIDE - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for hydrogen peroxide and what is the scope of patent protection?
Hydrogen peroxide
is the generic ingredient in one branded drug marketed by Aclaris and is included in one NDA. There are five patents protecting this compound. Additional information is available in the individual branded drug profile pages.Hydrogen peroxide has nineteen patent family members in sixteen countries.
There are four drug master file entries for hydrogen peroxide.
Summary for HYDROGEN PEROXIDE
| International Patents: | 19 |
| US Patents: | 5 |
| Tradenames: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Drug Master File Entries: | 4 |
| Raw Ingredient (Bulk) Api Vendors: | 83 |
| Clinical Trials: | 113 |
| Patent Applications: | 5,547 |
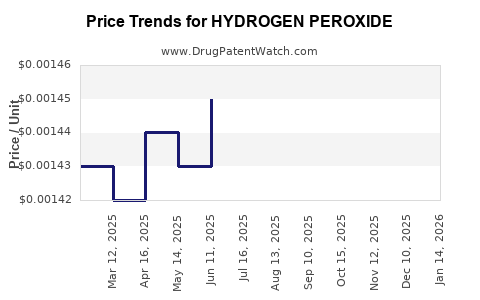
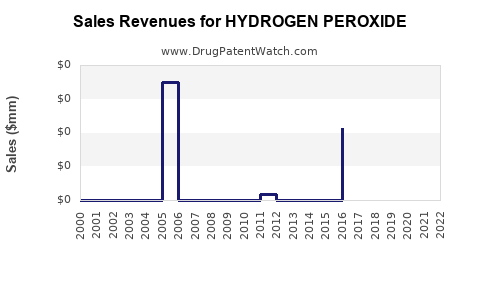
| Drug Prices: | Drug price trends for HYDROGEN PEROXIDE |
| What excipients (inactive ingredients) are in HYDROGEN PEROXIDE? | HYDROGEN PEROXIDE excipients list |
| DailyMed Link: | HYDROGEN PEROXIDE at DailyMed |
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for HYDROGEN PEROXIDE
Generic Entry Date for HYDROGEN PEROXIDE*:
Constraining patent/regulatory exclusivity:
Dosage:
SOLUTION;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for HYDROGEN PEROXIDE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Misr University for Science and Technology | PHASE1 |
| Universidade Federal do Para | PHASE1 |
| Mersin University | NA |
Medical Subject Heading (MeSH) Categories for HYDROGEN PEROXIDE
Anatomical Therapeutic Chemical (ATC) Classes for HYDROGEN PEROXIDE
US Patents and Regulatory Information for HYDROGEN PEROXIDE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | 9,980,983 | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | 9,675,639 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | 10,098,910 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | 10,493,103 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | DISCN | Yes | No | 10,729,720 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for HYDROGEN PEROXIDE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Aclaris | ESKATA | hydrogen peroxide | SOLUTION;TOPICAL | 209305-001 | Dec 14, 2017 | 7,381,427 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for HYDROGEN PEROXIDE
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Singapore | 11201608775X | ⤷ Get Started Free | |
| Mexico | 2016013826 | FORMULACIONES DE PEROXIDO Y METODOS Y APLICACIONES PARA UTILIZARLAS. (PEROXIDE FORMULATIONS AND METHODS AND APPLICATORS FOR USING THE SAME.) | ⤷ Get Started Free |
| South Korea | 20170029413 | 과산화물 제제 및 이를 사용하기 위한 방법 및 어플리케이터 (PEROXIDE FORMULATIONS AND METHODS AND APPLICATORS FOR USING THE SAME) | ⤷ Get Started Free |
| Mexico | 2016013826 | ⤷ Get Started Free | |
| Russian Federation | 2016145236 | ⤷ Get Started Free | |
| Denmark | 3134061 | ⤷ Get Started Free | |
| Australia | 2015249841 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for HYDROGEN PEROXIDE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0302769 | 98C0036 | Belgium | ⤷ Get Started Free | PRODUCT NAME: CLOPIDOGREL HYDROGENE SULFATE; REGISTRATION NO/DATE IN FRANCE: EU/1/98 /069/001 DU 19980715; REGISTRATION NO/DATE AT EEC: DU EU/1-/98/069/001 |
| 3106463 | 20C1012 | France | ⤷ Get Started Free | PRODUCT NAME: LAROTRECTINIB ET/OU SES SELS PHARMACEUTIQUEMENT ACCEPTABLES, EN PARTICULIER UN SULFATE DE LAROTRECTINIB TEL QUE L'HYDROGENOSULFATE DE LAROTRECTINIB; NAT. REGISTRATION NO/DATE: EU/1/19/1385 20190923; FIRST REGISTRATION: DE - EU/1/19/1385 20190923 |
| 1968948 | 2190048-5 | Sweden | ⤷ Get Started Free | PRODUCT NAME: SELUMETINIB HYDROGEN SULFATE, INCLUDING ANY SOLVATES AND ANHYDROUS FORMS THEREOF; REG. NO/DATE: EU/1/21/1552 20210619 |
| 0281459 | 98C0036 | France | ⤷ Get Started Free | PRODUCT NAME: CLOPIDOGREL HYDROGENE SULFATE; REGISTRATION NO/DATE IN FRANCE: EU/1/98 /069/001 DU 19980715; REGISTRATION NO/DATE AT EEC: DU EU/1-/98/069/001 |
| 1968948 | 2021C/549 | Belgium | ⤷ Get Started Free | PRODUCT NAME: SELUMETINIB (Y COMPRIS TOUS SELS PHARMACEUTIQUEMENT ACCEPTABLES (EN PARTICULIER HYDROGENOSULFATE), ESTERS, SOLVATES OU ENANTIOMERES DE CEUX-CI); AUTHORISATION NUMBER AND DATE: EU/1/21/1552 20210619 |
| 3106463 | CA 2020 00013 | Denmark | ⤷ Get Started Free | PRODUCT NAME: LAROTRECTINIB OG/ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, SAERLIGT LAROTRECTINIBSULFAT, INKLUSIV LAROTRECTINIBHYDROGENSULFAT; REG. NO/DATE: EU/1/19/1385 20190923 |
| 1968948 | CA 2021 00044 | Denmark | ⤷ Get Started Free | PRODUCT NAME: SELUMETINIB, INKLUSIV FARMACEUTISK ACCEPTABLE SALTE (ISAER HYDROGENSULFAT), ESTRE, SOLVATER OG ENANTIOMERE DERAF; REG. NO/DATE: EU/1/21/1552 20210619 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
More… ↓