ANASTROZOLE - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for anastrozole and what is the scope of freedom to operate?
Anastrozole
is the generic ingredient in two branded drugs marketed by Accord Hlthcare, Apotex Inc, Beijing Yiling, Chartwell Molecular, Chartwell Rx, Cipla, Eugia Pharma, Fresenius Kabi Usa, Hikma, Impax Labs Inc, Kenton, Natco Pharma Ltd, Pharmobedient, Sandoz, Sun Pharm Inds Ltd, Synthon Pharms, Teva Pharms, Watson Labs Teva, Zydus Pharms Usa Inc, and Ani Pharms, and is included in twenty NDAs. Additional information is available in the individual branded drug profile pages.There are twenty-four drug master file entries for anastrozole. Twenty-five suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for ANASTROZOLE
| US Patents: | 0 |
| Tradenames: | 2 |
| Applicants: | 20 |
| NDAs: | 20 |
| Drug Master File Entries: | 24 |
| Finished Product Suppliers / Packagers: | 25 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 329 |
| Patent Applications: | 6,707 |
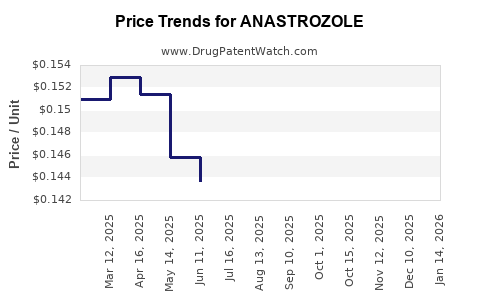
| Drug Prices: | Drug price trends for ANASTROZOLE |
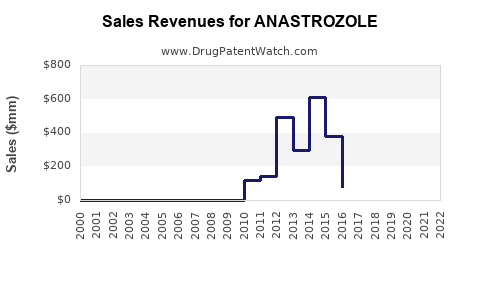
| Drug Sales Revenues: | Drug sales revenues for ANASTROZOLE |
| What excipients (inactive ingredients) are in ANASTROZOLE? | ANASTROZOLE excipients list |
| DailyMed Link: | ANASTROZOLE at DailyMed |
Recent Clinical Trials for ANASTROZOLE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Nagoya City University | PHASE1 |
| Eli Lilly and Company | PHASE3 |
| Novartis | PHASE1 |
Generic filers with tentative approvals for ANASTROZOLE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Get Started Free | ⤷ Get Started Free | 1MG | TABLET;ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for ANASTROZOLE
| Drug Class | Aromatase Inhibitor |
| Mechanism of Action | Aromatase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ANASTROZOLE
US Patents and Regulatory Information for ANASTROZOLE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmobedient | ANASTROZOLE | anastrozole | TABLET;ORAL | 091051-001 | Jun 28, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Sun Pharm Inds Ltd | ANASTROZOLE | anastrozole | TABLET;ORAL | 091177-001 | Jul 15, 2011 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Kenton | ANASTROZOLE | anastrozole | TABLET;ORAL | 078944-001 | Jun 28, 2010 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Fresenius Kabi Usa | ANASTROZOLE | anastrozole | TABLET;ORAL | 090088-001 | Jun 28, 2010 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ANASTROZOLE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ani Pharms | ARIMIDEX | anastrozole | TABLET;ORAL | 020541-001 | Dec 27, 1995 | 4,935,437 | ⤷ Get Started Free |
| Ani Pharms | ARIMIDEX | anastrozole | TABLET;ORAL | 020541-001 | Dec 27, 1995 | RE36617*PED | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Market Dynamics and Financial Trajectory for Anastrozole
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.