ENBREL Drug Profile
✉ Email this page to a colleague
Summary for Tradename: ENBREL
| High Confidence Patents: | 13 |
| Applicants: | 1 |
| BLAs: | 1 |
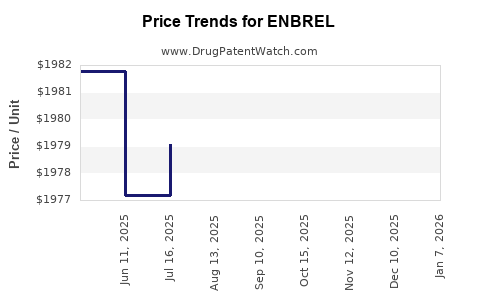
| Drug Prices: | Drug price information for ENBREL |
| Recent Clinical Trials: | See clinical trials for ENBREL |
Recent Clinical Trials for ENBREL
Identify potential brand extensions & biosimilar entrants
| Sponsor | Phase |
|---|---|
| mAbxience Research S.L. | PHASE3 |
| mAbxience Research S.L. | PHASE1 |
| Benaroya Research Institute | Early Phase 1 |
Pharmacology for ENBREL
| Mechanism of Action | Tumor Necrosis Factor Receptor Blocking Activity |
| Established Pharmacologic Class | Tumor Necrosis Factor Blocker |
Note on Biologic Patents
Matching patents to biologic drugs is far more complicated than for small-molecule drugs.
DrugPatentWatch employs three methods to identify biologic patents:
- Brand-side disclosures in response to biosimilar applications
- DrugPatentWatch analysis and company disclosures
- Patents from broad patent text search
These patents were identified from disclosures by the brand-side company, in response to a potential biosimilar seeking to launch. They have a high certainty of blocking biosimilar entry. The expiration dates listed are not estimates — they're expiration dates as indicated by the brand-side company.
These patents were identified from searching various sources, including drug labels and other general disclosures from the brand-side company. This list may exclude some of the patents which block biosimilar launch, and some of these patents listed may not actually block biosimilar launch. The expiration dates listed for these patents are estimates, based on the grant date of the patent.
For completeness, these patents were identified by searching the patent literature for mentions of the branded or ingredient name of the drug. Some of these patents protect the original drug, whereas others may protect follow-on inventions or even inventions casually mentioning the drug. The expiration dates listed for these patents are estimates, based on the grant date of the patent.
1) High Certainty: US Patents for ENBREL Derived from Brand-Side Litigation
No patents found based on brand-side litigation
2) High Certainty: US Patents for ENBREL Derived from DrugPatentWatch Analysis and Company Disclosures
| Applicant | Tradename | Biologic Ingredient | Dosage Form | BLA | Patent No. | Estimated Patent Expiration | Source |
|---|---|---|---|---|---|---|---|
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 10,307,483 | 2038-04-20 | DrugPatentWatch analysis and company disclosures |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 11,491,223 | 2038-09-27 | DrugPatentWatch analysis and company disclosures |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 5,395,760 | 2010-05-10 | DrugPatentWatch analysis and company disclosures |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 5,605,690 | 2015-02-08 | DrugPatentWatch analysis and company disclosures |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 5,712,155 | 2014-11-29 | DrugPatentWatch analysis and company disclosures |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 5,945,397 | 2016-05-16 | DrugPatentWatch analysis and company disclosures |
| >Applicant | >Tradename | >Biologic Ingredient | >Dosage Form | >BLA | >Patent No. | >Estimated Patent Expiration | >Source |
3) Low Certainty: US Patents for ENBREL Derived from Patent Text Search
| Applicant | Tradename | Biologic Ingredient | Dosage Form | BLA | Patent No. | Estimated Patent Expiration | Source |
|---|---|---|---|---|---|---|---|
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 10,010,585 | 2035-06-16 | Patent claims search |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 10,016,338 | 2036-12-20 | Patent claims search |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 10,028,940 | 2037-03-16 | Patent claims search |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 10,047,141 | 2035-01-29 | Patent claims search |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 10,058,589 | 2033-10-24 | Patent claims search |
| Immunex Corporation | ENBREL | etanercept | For Injection | 103795 | 10,080,734 | 2037-10-24 | Patent claims search |
| >Applicant | >Tradename | >Biologic Ingredient | >Dosage Form | >BLA | >Patent No. | >Estimated Patent Expiration | >Source |
International Patents for ENBREL
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2721745 | ⤷ Get Started Free |
| Norway | 312769 | ⤷ Get Started Free |
| Netherlands | 300008 | ⤷ Get Started Free |
| Australia | 630497 | ⤷ Get Started Free |
| Japan | 2960039 | ⤷ Get Started Free |
| Austria | E131871 | ⤷ Get Started Free |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for ENBREL
| Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|
| 15/2000 | Austria | ⤷ Get Started Free | PRODUCT NAME: ETANERCEPT; NAT. REGISTRATION NO/DATE: EU/1/99/126/001 20000203; FIRST REGISTRATION: LI 55365 20000201 |
| 10075011 | Germany | ⤷ Get Started Free | PRODUCT NAME: ENBREL-ETANERCEPT; NAT. REGISTRATION NO/DATE: EU/1/99/126/001 20000203; FIRST REGISTRATION: CH (LI) 55365 20000201 |
| 00C0015 | France | ⤷ Get Started Free | PRODUCT NAME: ETANERCEPT; NAT. REGISTRATION NO/DATE: EU/1/99/126/001 20000203; FIRST REGISTRATION: IKS- N 55365 20000201 |
| C00939121/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: ETANERCEPT; REGISTRATION NUMBER/DATE: IKS 55365 01.02.2000 |
| 132000900852970 | Italy | ⤷ Get Started Free | |
| 10399023 | Germany | ⤷ Get Started Free | PRODUCT NAME: ETANERCEPT; REGISTRATION NO/DATE: EU/1/99/126/001-002 20000203 |
| >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Biologic Drug: ENBREL
More… ↓