Vertex Pharms Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for VERTEX PHARMS INC, and when can generic versions of VERTEX PHARMS INC drugs launch?
VERTEX PHARMS INC has six approved drugs.
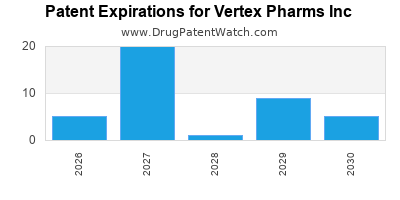
There are forty-seven US patents protecting VERTEX PHARMS INC drugs.
There are six hundred and sixty-one patent family members on VERTEX PHARMS INC drugs in forty-seven countries and sixty-eight supplementary protection certificates in nineteen countries.
Summary for Vertex Pharms Inc
| International Patents: | 661 |
| US Patents: | 47 |
| Tradenames: | 4 |
| Ingredients: | 4 |
| NDAs: | 6 |
Drugs and US Patents for Vertex Pharms Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vertex Pharms Inc | TRIKAFTA (COPACKAGED) | elexacaftor, ivacaftor, tezacaftor; ivacaftor | TABLET;ORAL | 212273-001 | Oct 21, 2019 | RX | Yes | Yes | 10,081,621 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Vertex Pharms Inc | TRIKAFTA (COPACKAGED) | elexacaftor, ivacaftor, tezacaftor; ivacaftor | TABLET;ORAL | 212273-002 | Jun 8, 2021 | RX | Yes | No | 11,578,062 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Vertex Pharms Inc | TRIKAFTA (COPACKAGED) | elexacaftor, ivacaftor, tezacaftor; ivacaftor | GRANULES;ORAL | 217660-002 | Apr 26, 2023 | RX | Yes | Yes | 11,147,770 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Vertex Pharms Inc | SYMDEKO (COPACKAGED) | ivacaftor; ivacaftor, tezacaftor | TABLET;ORAL | 210491-002 | Jun 21, 2019 | RX | Yes | No | 10,058,546 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Vertex Pharms Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 1773816 | ⤷ Try a Trial |
| Denmark | 3170818 | ⤷ Try a Trial |
| South Korea | 20080066086 | ⤷ Try a Trial |
| South Africa | 200700601 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Vertex Pharms Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3170818 | SPC/GB20/041 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF (A) 3-(6-(1-(2,2-DIFLUOROBENZO(D)(1,3)DIOXOL-5-YL)CYCLOPROPANECARBOXAMIDO)-3-METHYLPYRIDIN-2-YL)BENZOIC ACID (I.E. LUMACAFTOR) AND (B) N-(5-HYDROXY-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE (I.E. IVACAFTOR) OR A PHARMACEUT; REGISTERED: UK EU/1/15/1059(NI) 20151124; UK PLGB 22352/0004 20151124 |
| 2826776 | CA 2021 00013 | Denmark | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF (A) (R)-1-(2,2-DIFLUOROBENZO(D)(1,3)DIOXOL-5-YL)-N-(1-(2,3-DIHYDROXYPROPYL)-6-FLUORO-2-(1-HYDROXY-2-METHYLPROPAN-2-YL)-1H-INDOL-5-YL)CYCLOPROPANECARBOXAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND (B) ....; REG. NO/DATE: EU/1/18/1306 20181106 |
| 2404919 | 17/2016 | Austria | ⤷ Try a Trial | PRODUCT NAME: LUMACAFTOR ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON ODER EIN ESTER PRODRUG DAVON; REGISTRATION NO/DATE: EU/1/15/1059 (MITTEILUNG) 20151124 |
| 3170818 | 2090033-8 | Sweden | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF (A) 3-(6-(1-(2,2-DIFLUOROBENZOD1,3DIOXOL-5-YL)CYCLOPROPANECARBOXAMIDO)-3-METHYLPYRIDIN-2-YL)BENZOIC ACID AND (B) N-(5-HYDROXY-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REG. NO/DATE: EU/1/15/1059 20151124 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.