Novo Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NOVO, and when can generic versions of NOVO drugs launch?
NOVO has eleven approved drugs.
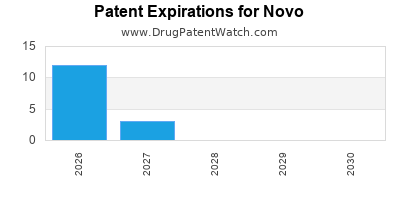
There are fifty-six US patents protecting NOVO drugs.
There are four hundred and twenty-two patent family members on NOVO drugs in forty countries and one hundred and nineteen supplementary protection certificates in seventeen countries.
Drugs and US Patents for Novo
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo | RYBELSUS | semaglutide | TABLET;ORAL | 213051-003 | Sep 20, 2019 | RX | Yes | Yes | 8,129,343 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-004 | Oct 6, 2022 | RX | Yes | Yes | 9,687,611 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novo | WEGOVY | semaglutide | SOLUTION;SUBCUTANEOUS | 215256-002 | Jun 4, 2021 | RX | Yes | Yes | 11,752,198 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novo | WEGOVY | semaglutide | SOLUTION;SUBCUTANEOUS | 215256-005 | Jun 4, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-003 | Mar 28, 2022 | RX | Yes | Yes | 8,114,833 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novo | WEGOVY | semaglutide | SOLUTION;SUBCUTANEOUS | 215256-005 | Jun 4, 2021 | RX | Yes | Yes | 8,129,343 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Novo | RIVFLOZA | nedosiran sodium | SOLUTION;INJECTION | 215842-001 | Sep 29, 2023 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Novo
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | PRANDIMET | metformin hydrochloride; repaglinide | TABLET;ORAL | 022386-001 | Jun 23, 2008 | 6,677,358 | ⤷ Sign Up |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-001 | Dec 5, 2017 | 8,672,898 | ⤷ Sign Up |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-004 | Oct 6, 2022 | 7,762,994 | ⤷ Sign Up |
| Novo Nordisk Inc | PRANDIMET | metformin hydrochloride; repaglinide | TABLET;ORAL | 022386-002 | Jun 23, 2008 | 6,677,358 | ⤷ Sign Up |
| Novo | MACRILEN | macimorelin acetate | FOR SOLUTION;ORAL | 205598-001 | Dec 20, 2017 | 6,861,409 | ⤷ Sign Up |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | 7,762,994 | ⤷ Sign Up |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | 8,579,869 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 1 mg/500 mg and 2 mg/500 mg | ➤ Subscribe | 2009-04-09 |
| ➤ Subscribe | Injection | 18 mg/3 mL prefilled syringe | ➤ Subscribe | 2016-12-12 |
| ➤ Subscribe | Vaginal Tablets | 10 mcg | ➤ Subscribe | 2013-01-02 |
International Patents for Novo Drugs
Supplementary Protection Certificates for Novo Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1412357 | 122008000046 | Germany | ⤷ Sign Up | PRODUCT NAME: SITAGLIPTIN, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, INSBESONDERE SITAGLIPTINPHOSPHAT- MONOHYDRAT, IN KOMBINATION MIT METFORMIN, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, INSBESONDERE DES HYDROCHLORIDS; NAT. REGISTRATION NO/DATE: EU/1/08/455/001-014 20080716 FIRST REGISTRATION: CH/LI 58450 01 58450 02 58450 03 20080408 |
| 2498758 | PA2020003 | Lithuania | ⤷ Sign Up | PRODUCT NAME: METFORMINAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; SAKSAGLIPTINAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; DAPAGLIFLOZINAS ARBA FARMACINIU POZIURIU PRIIMTINAS JO SOLVATAS; REGISTRATION NO/DATE: EU/1/19/1401 20191111 |
| 1412357 | CA 2008 00035 | Denmark | ⤷ Sign Up | PRODUCT NAME: SITAGLIPTIN VALGFRIT I FORM AF ET FARMACEUTISK ACCEPTABELT SALT, ISAER MONOPHOSPHAT, METFORMIN VALGFRIT I FORM AF ET FARMACEUTISK ACCEPTABELT SALT, ISAER HYDROCHLORID |
| 1863839 | 2018/017 | Ireland | ⤷ Sign Up | PRODUCT NAME: OZEMPIC-SEMAGLUTIDE; REGISTRATION NO/DATE: EU/1/17/1251 20180208 |
| 1863839 | C201830026 | Spain | ⤷ Sign Up | PRODUCT NAME: OZEMPIC-SEMAGLUTIDA; NATIONAL AUTHORISATION NUMBER: EU/1/17/1251; DATE OF AUTHORISATION: 20180208; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/17/1251; DATE OF FIRST AUTHORISATION IN EEA: 20180208 |
| 2209800 | 1490067-4 | Sweden | ⤷ Sign Up | PRODUCT NAME: COMBINATION OF INSULIN DEGLUDEC AND LIRAGLUTIDE; REG. NO/DATE: EU/1/14/974 20140918 |
| 1412357 | PA2008013 | Lithuania | ⤷ Sign Up | PRODUCT NAME: SITAGLIPTINUM PHOSPHAS MONOHYDRICUS, METFORMINI HYDROCHLORIDUM; REG. NO/DATE: EU/1/08/455/001-014 20080716 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.