NOVEN Company Profile
✉ Email this page to a colleague
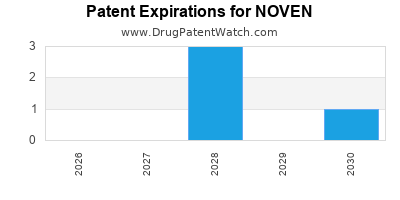
What is the competitive landscape for NOVEN, and when can generic versions of NOVEN drugs launch?
NOVEN has eight approved drugs.
There are eleven US patents protecting NOVEN drugs. There are three tentative approvals on NOVEN drugs.
There are fifty-four patent family members on NOVEN drugs in eighteen countries and fifty-three supplementary protection certificates in fourteen countries.
Summary for NOVEN
| International Patents: | 54 |
| US Patents: | 11 |
| Tradenames: | 11 |
| Ingredients: | 7 |
| NDAs: | 8 |
| Patent Litigation for NOVEN: | See patent lawsuits for NOVEN |
| PTAB Cases with NOVEN as petitioner: | See PTAB cases with NOVEN as petitioner |
Drugs and US Patents for NOVEN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-004 | Mar 22, 2022 | RX | Yes | Yes | 8,591,941 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | AB3 | RX | Yes | No | 9,730,900 | ⤷ Sign Up | ⤷ Sign Up | |||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-003 | Mar 22, 2022 | RX | Yes | No | 8,632,802 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | AB3 | RX | Yes | No | 9,833,419 | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NOVEN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-002 | Oct 29, 2012 | 6,024,976 | ⤷ Sign Up |
| Noven Pharms Inc | COMBIPATCH | estradiol; norethindrone acetate | FILM, EXTENDED RELEASE;TRANSDERMAL | 020870-001 | Aug 7, 1998 | 5,656,286 | ⤷ Sign Up |
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-003 | Apr 6, 2006 | 6,210,705 | ⤷ Sign Up |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-002 | Oct 29, 2012 | 6,841,716 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVEN drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Transdermal System | 0.0375 mg/day, 0.05 mg/day, 0.075 mg/day, 0.1 mg/day | ➤ Subscribe | 2014-08-18 |
| ➤ Subscribe | Transdermal System | 10 mg/9 hrs, 15 mg/9 hrs, 20 mg/9 hrs and 30 mg/9 hrs | ➤ Subscribe | 2011-04-13 |
| ➤ Subscribe | Transdermal System | 0.025 mg/day | ➤ Subscribe | 2015-05-08 |
International Patents for NOVEN Drugs
Supplementary Protection Certificates for NOVEN Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0334429 | 97C0002 | Belgium | ⤷ Sign Up | PRODUCT NAME: ESTRADIOL; NAT. REGISTRATION NO/DATE: NL 18978 19960731; FIRST REGISTRATION: SE - 11783 19930305 |
| 0285237 | 95C0008 | Belgium | ⤷ Sign Up | PRODUCT NAME: ESTRADIOL, HEMIHYDRATE; NAT. REGISTRATION NO/DATE: NL 19489 19941107; FIRST REGISTRATION: FR - NL 19489 19941107 |
| 1453521 | 93156 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL ET ETHINYLESTRADIOL; FIRST REGISTRATION DATE: 20150211 |
| 1453521 | 122015000093 | Germany | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 87675.00.00 20150720; FIRST REGISTRATION: SLOWAKEI 17/0017/15-S 20150129 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.