XELJANZ Drug Patent Profile
✉ Email this page to a colleague
When do Xeljanz patents expire, and what generic alternatives are available?
Xeljanz is a drug marketed by Pfizer and Pf Prism Cv and is included in three NDAs. There are four patents protecting this drug and three Paragraph IV challenges.
This drug has seventy-five patent family members in fifty-two countries.
The generic ingredient in XELJANZ is tofacitinib citrate. There are twelve drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the tofacitinib citrate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Xeljanz
A generic version of XELJANZ was approved as tofacitinib citrate by HIKMA on September 25th, 2023.
Summary for XELJANZ
| International Patents: | 75 |
| US Patents: | 1 |
| Applicants: | 2 |
| NDAs: | 3 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 90 |
| Clinical Trials: | 30 |
| Patent Applications: | 106 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for XELJANZ |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for XELJANZ |
| What excipients (inactive ingredients) are in XELJANZ? | XELJANZ excipients list |
| DailyMed Link: | XELJANZ at DailyMed |
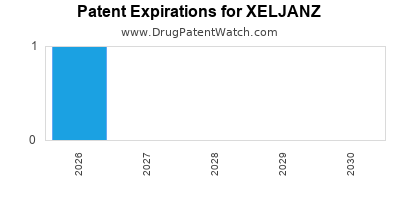
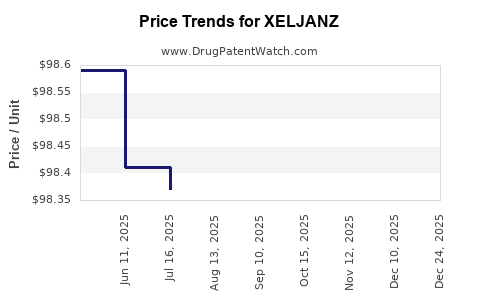
Recent Clinical Trials for XELJANZ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Takeda | Phase 4 |
| Children's Hospital Los Angeles | Phase 2 |
| Fundación de Investigación Biomédica - Hospital Universitario de La Princesa | Phase 4 |
Pharmacology for XELJANZ
| Drug Class | Janus Kinase Inhibitor |
| Mechanism of Action | Janus Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for XELJANZ
Paragraph IV (Patent) Challenges for XELJANZ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| XELJANZ | Oral Solution | tofacitinib citrate | 1 mg/mL | 213082 | 1 | 2021-11-12 |
| XELJANZ | Tablets | tofacitinib citrate | 10 mg | 203214 | 1 | 2019-07-24 |
| XELJANZ | Tablets | tofacitinib citrate | 5 mg | 203214 | 3 | 2016-11-07 |
US Patents and Regulatory Information for XELJANZ
XELJANZ is protected by one US patents and one FDA Regulatory Exclusivity.
Patents protecting XELJANZ
Pyrrolo[2,3-D]pyrimidine compounds
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting XELJANZ
TREATMENT OF ADULT PATIENTS WITH ACTIVE ANKYLOSING SPONDYLITIS WHO HAVE HAD AN INADEQUATE RESPONSE OR INTOLERANCE TO ONE OR MORE TNF BLOCKERS, TO THE PRESCRIBING INFORMATION
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | XELJANZ | tofacitinib citrate | SOLUTION;ORAL | 213082-001 | Sep 25, 2020 | AA | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-002 | Dec 12, 2019 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-002 | May 30, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-001 | Nov 6, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-001 | Nov 6, 2012 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Pfizer | XELJANZ XR | tofacitinib citrate | TABLET, EXTENDED RELEASE;ORAL | 208246-001 | Feb 23, 2016 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for XELJANZ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-002 | May 30, 2018 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-002 | May 30, 2018 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | XELJANZ | tofacitinib citrate | SOLUTION;ORAL | 213082-001 | Sep 25, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-001 | Nov 6, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| Pfizer | XELJANZ | tofacitinib citrate | SOLUTION;ORAL | 213082-001 | Sep 25, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-002 | May 30, 2018 | ⤷ Sign Up | ⤷ Sign Up |
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-001 | Nov 6, 2012 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for XELJANZ
See the table below for patents covering XELJANZ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Georgia, Republic of | P20063784 | Optical Resolution of (1-Benzyl-4- Methylpiperidin-3-Yl) -Methylamine Enantiomers and Use Thereof for Preparation of Pyrrolo 2,3-Pyrimidine Derivatives as Protein Kinases Inhibitors | ⤷ Sign Up |
| Spain | 2295495 | ⤷ Sign Up | |
| South Korea | 20060133117 | ⤷ Sign Up | |
| Czech Republic | 20033260 | ⤷ Sign Up | |
| Australia | 2008203170 | ⤷ Sign Up | |
| Eurasian Patent Organization | 012666 | ОПТИЧЕСКОЕ РАЗДЕЛЕНИЕ (1-БЕНЗИЛ-4-МЕТИЛПИПЕРИДИН-3-ИЛ)МЕТИЛАМИНА И ЕГО ПРИМЕНЕНИЕ ДЛЯ ПОЛУЧЕНИЯ ПРОИЗВОДНЫХ ПИРРОЛО 2,3-ПИРИМИДИНА В КАЧЕСТВЕ ИНГИБИТОРОВ ПРОТЕИНКИНАЗЫ (OPTICAL RESOLUTION OF (1-BENZYL-4-METHYLPIPERIDIN-3-YL)-METHYLAMINE AND THE USE THEREOF FOR THE PREPARATION OF PYRROLO 2,3-PYRIMIDINE DERIVATIVES AS PROTEIN KINASES INHIBITORS) | ⤷ Sign Up |
| Germany | 60007552 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for XELJANZ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1666481 | C 2017 029 | Romania | ⤷ Sign Up | PRODUCT NAME: TOFACITINIB, OPTIONAL SUB FORMA UNEI SARI ACCEPTABILE FARMACEUTIC, INCLUSIV SAREA CITRAT 3-((3R,4R)-4-METIL-3-{METIL-(7H-PIROLO[2,3-D]PIRIMIDIN-4-IL)-AMINO]PERIDINIL)-3-OXO-PROPIONITRIL; NATIONAL AUTHORISATION NUMBER: EU/1/17/1178; DATE OF NATIONAL AUTHORISATION: 20170322; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/17/1178; DATE OF FIRST AUTHORISATION IN EEA: 20170322 |
| 1666481 | CA 2017 00035 | Denmark | ⤷ Sign Up | PRODUCT NAME: TOFACITINIB, EVENTUELT I FORM AF ET FARMACEUTISK ACCEPTABELT SALT, HERUNDER CITRAT-SALTET; REG. NO/DATE: EU/1/17/1178/001-003 20170324 |
| 1666481 | 132017000095300 | Italy | ⤷ Sign Up | PRODUCT NAME: TOFACITINIB, OPZIONALMENTE NELLA FORMA DI UN SALE FARMACEUTICAMENTE ACCETTABILE, COMPRENDENTE IL SALE CITRATO(XELJANZ); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/17/1178/001-004, 20170324 |
| 1666481 | 2017/036 | Ireland | ⤷ Sign Up | PRODUCT NAME: TOFACITINIB, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE CITRATE SALT; REGISTRATION NO/DATE: EU/1/17/1178/001 EU/1/17/1178/004 20170322 |
| 1666481 | 2017C/032 | Belgium | ⤷ Sign Up | PRODUCT NAME: TOFACITINIB; AUTHORISATION NUMBER AND DATE: EU/1/17/1178 20170324 |
| 1666481 | 300887 | Netherlands | ⤷ Sign Up | PRODUCT NAME: TOFACITINIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER TOFACITINIBCITRAAT; REGISTRATION NO/DATE: EU/1/17/1178 20170324 |
| 1666481 | CR 2017 00035 | Denmark | ⤷ Sign Up | PRODUCT NAME: TOFACITINIB, EVENTUELT I FORM AF ET FARMACEUTISK ACCEPTABELT SALT, HERUNDER CITRAT-SALTET; REG. NO/DATE: EU/1/17/1178/001-003 20170324 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


