VITAMIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Vitamin, and when can generic versions of Vitamin launch?
Vitamin is a drug marketed by Banner Pharmacaps, Bristol Myers Squibb, Chase Chem, Elkins Sinn, Everylife, Impax Labs, Ivax Sub Teva Pharms, Mk Labs, West Ward, Wharton Labs, Arcum, Bel Mar, Teva, Bionpharma, Chartwell Molecular, Vitarine, and Hospira. and is included in thirty-five NDAs.
The generic ingredient in VITAMIN is phytonadione. There are ten drug master file entries for this compound. Seventeen suppliers are listed for this compound. Additional details are available on the phytonadione profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Vitamin
A generic version of VITAMIN was approved as phytonadione by AMNEAL PHARMS CO on May 11th, 2018.
Summary for VITAMIN
| US Patents: | 0 |
| Applicants: | 17 |
| NDAs: | 35 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for VITAMIN |
| Drug Sales Revenues: | Drug sales revenues for VITAMIN |
| DailyMed Link: | VITAMIN at DailyMed |
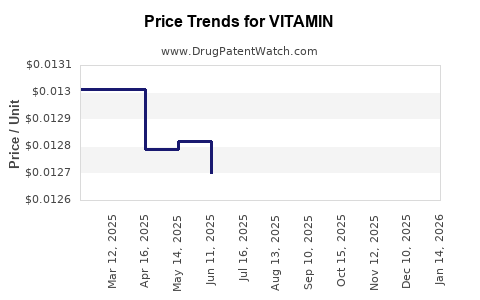
US Patents and Regulatory Information for VITAMIN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitarine | VITAMIN D | ergocalciferol | CAPSULE;ORAL | 084053-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Chartwell Molecular | VITAMIN D | ergocalciferol | CAPSULE;ORAL | 080825-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| West Ward | VITAMIN D | ergocalciferol | CAPSULE;ORAL | 083102-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


