VICTOZA Drug Patent Profile
✉ Email this page to a colleague
When do Victoza patents expire, and when can generic versions of Victoza launch?
Victoza is a drug marketed by Novo Nordisk Inc and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred patent family members in twenty-nine countries.
The generic ingredient in VICTOZA is liraglutide. There are seven drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the liraglutide profile page.
DrugPatentWatch® Generic Entry Outlook for Victoza
Victoza was eligible for patent challenges on January 25, 2014.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 13, 2026. This may change due to patent challenges or generic licensing.
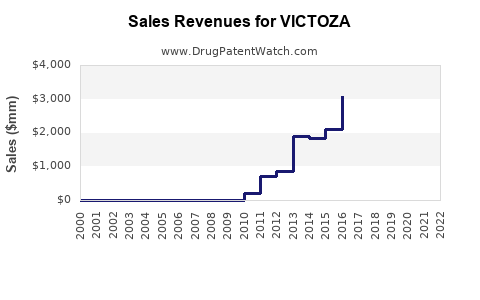
Annual sales in 2021 were $3.4bn, indicating a strong incentive for generic entry (peak sales were $5.6bn in 2019).
There have been twenty patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (liraglutide), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for VICTOZA?
- What are the global sales for VICTOZA?
- What is Average Wholesale Price for VICTOZA?
Summary for VICTOZA
| International Patents: | 100 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 27 |
| Clinical Trials: | 126 |
| Patent Applications: | 2,997 |
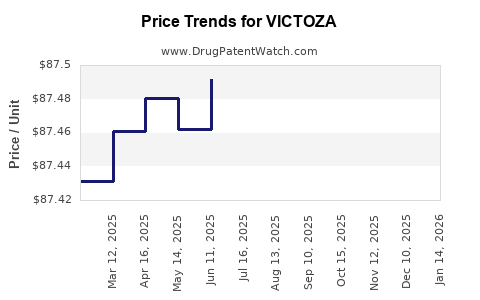
| Drug Prices: | Drug price information for VICTOZA |
| Drug Sales Revenues: | Drug sales revenues for VICTOZA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for VICTOZA |
| What excipients (inactive ingredients) are in VICTOZA? | VICTOZA excipients list |
| DailyMed Link: | VICTOZA at DailyMed |
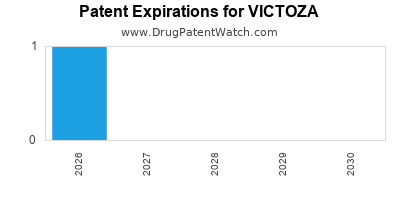
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VICTOZA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VICTOZA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Chicago | Early Phase 1 |
| Carmot Therapeutics, Inc. | Phase 1 |
| Yale University | Phase 3 |
Pharmacology for VICTOZA
| Drug Class | GLP-1 Receptor Agonist |
| Mechanism of Action | Glucagon-like Peptide-1 (GLP-1) Agonists |
Paragraph IV (Patent) Challenges for VICTOZA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| VICTOZA | Injection | liraglutide | 18 mg/3 mL prefilled syringe | 022341 | 1 | 2016-12-12 |
US Patents and Regulatory Information for VICTOZA
VICTOZA is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VICTOZA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting VICTOZA
Needle mounting system and a method for mounting a needle assembly
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Propylene glycol-containing peptide formulations which are optimal for production and for use in injection devices
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Injection button
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Liraglutide in cardiovascular conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VICTOZA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for VICTOZA
See the table below for patents covering VICTOZA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Germany | 69900026 | ⤷ Sign Up | |
| Australia | 6820500 | ⤷ Sign Up | |
| Japan | 2012188424 | PROPYLENE GLYCOL-CONTAINING PEPTIDE FORMULATION OPTIMAL FOR PRODUCTION AND USE IN INJECTION DEVICE | ⤷ Sign Up |
| Turkey | 201906789 | ⤷ Sign Up | |
| China | 108778317 | 用于心血管病况的利拉鲁肽 (LIRAGLUTIDE IN CARDIOVASCULAR CONDITIONS) | ⤷ Sign Up |
| Ukraine | 72181 | Translated By PlajПРОИЗВОДНЫЕ ГЛЮКАГОНООБРАЗНОГО ПЕПТИДА-1;ПОХІДНА ГЛЮКАГОНОПОДІБНОГО ПЕПТИДУ-1 (ВАРІАНТИ), ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ ТА СПОСІБ ОДЕРЖАННЯ ЛІКУВАЛЬНОГО ЗАСОБУ (ВАРІАНТИ), СПОСІБ ЛІКУВАННЯ ДІАБЕТУ (ВАРІАНТИ) І ОЖИРІННЯ (DERIVATIVES OF GLUCANOLIKE PEPTIDE-1) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VICTOZA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2209800 | SPC/GB14/079 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: COMBINATION OF INSULIN DEGLUDEC, OR PHARMACEUTICALLY ACCEPTABLE SOLVATES, HYDRATES, OR SALTS THEREOF, AND LIRAGLUTIDE, OR PHARMACEUTICALLY ACCEPTABLE SOLVATES, HYDRATES, OR SALTS THEREOF; REGISTERED: CH 65041 20140912; UK EU/1/14/947 20140922 |
| 2209800 | 132014902311502 | Italy | ⤷ Sign Up | PRODUCT NAME: INSULINA DEGLUDEC/LIRAGLUTIDE(XULTOPHY); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/14/947, 20140918 |
| 0944648 | CA 2009 00041 | Denmark | ⤷ Sign Up | |
| 0944648 | C00944648/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: LIRAGLUTID; REGISTRATION NO/DATE: SWISSMEDIC 59329 11.12.2009 |
| 0944648 | 09C0054 | France | ⤷ Sign Up | PRODUCT NAME: LIRAGLUTIDE; REGISTRATION NO/DATE IN FRANCE: EU/1/09/529/001 DU 20090630; REGISTRATION NO/DATE AT EEC: EU/1/09/529/001 DU 20090630 |
| 0944648 | SPC/GB09/058 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: LIRAGLUTIDE; REGISTERED: UK EU/1/09/529/001 20090630 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


