VARENICLINE Drug Patent Profile
✉ Email this page to a colleague
When do Varenicline patents expire, and what generic alternatives are available?
Varenicline is a drug marketed by Ajanta Pharma Ltd, Alkem Labs Ltd, Apotex, Aurobindo Pharma, Dr Reddys, Hetero Labs Ltd Iii, Kanchan Hlthcare, Lupin Ltd, Macleods Pharms Ltd, Mankind Pharma, Micro Labs, Ne Rx Pharma, Ph Health, Pharmobedient, Piramal, Regcon Holdings, Rhodes Pharms, Shilpa, Viwit Pharm, and Zydus. and is included in twenty NDAs.
The generic ingredient in VARENICLINE is varenicline tartrate. There are twelve drug master file entries for this compound. Twenty-nine suppliers are listed for this compound. Additional details are available on the varenicline tartrate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Varenicline
A generic version of VARENICLINE was approved as varenicline tartrate by PH HEALTH on August 11th, 2021.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for VARENICLINE?
- What are the global sales for VARENICLINE?
- What is Average Wholesale Price for VARENICLINE?
Summary for VARENICLINE
| US Patents: | 0 |
| Applicants: | 20 |
| NDAs: | 20 |
| Drug Prices: | Drug price information for VARENICLINE |
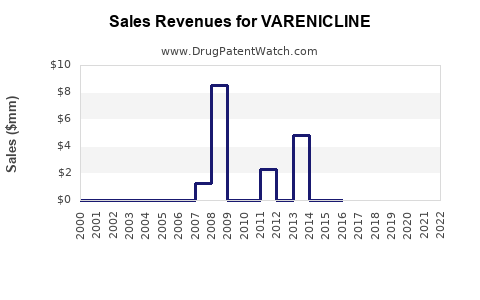
| Drug Sales Revenues: | Drug sales revenues for VARENICLINE |
| DailyMed Link: | VARENICLINE at DailyMed |
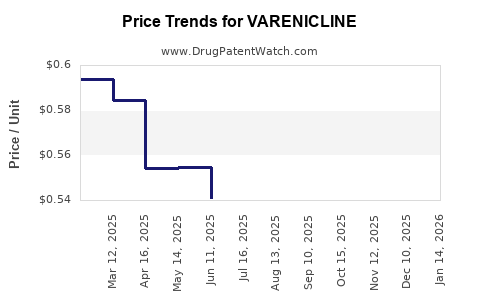
See drug prices for VARENICLINE
Recent Clinical Trials for VARENICLINE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| VA Office of Research and Development | PHASE4 |
| Sidney Kimmel Cancer Center at Thomas Jefferson University | PHASE2 |
| National Cancer Institute (NCI) | PHASE2 |
Anatomical Therapeutic Chemical (ATC) Classes for VARENICLINE
US Patents and Regulatory Information for VARENICLINE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regcon Holdings | VARENICLINE TARTRATE | varenicline tartrate | TABLET;ORAL | 219106-002 | Oct 29, 2024 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Pharmobedient | VARENICLINE TARTRATE | varenicline tartrate | TABLET;ORAL | 202019-002 | Feb 28, 2024 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Rhodes Pharms | VARENICLINE TARTRATE | varenicline tartrate | TABLET;ORAL | 213268-001 | May 21, 2025 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Piramal | VARENICLINE TARTRATE | varenicline tartrate | TABLET;ORAL | 217115-001 | Jul 23, 2024 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
