VANCOMYCIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Vancomycin, and what generic alternatives are available?
Vancomycin is a drug marketed by Xellia Pharms Aps, Fresenius Kabi Usa, Lupin Ltd, Orient Pharma Co Ltd, Pai Holdings Pharm, Strides Pharma, Watson Labs, Alkem Labs Ltd, Amneal, Aspiro, Avet Lifesciences, Eugia Pharma, Gland Pharma Ltd, Hainan Poly, Hainan Poly Pharm, Hikma, Hikma Pharms, Hospira, Hospira Inc, Knack, Meitheal, Mylan Labs Ltd, Pharm Assoc, Sagent Pharms, Sandoz, Sandoz Inc, Teva Pharms Usa, Zhejiang Novus Pharm, Samson Medcl, and Baxter Hlthcare. and is included in fifty-four NDAs. There are six patents protecting this drug.
This drug has forty-seven patent family members in twenty-nine countries.
The generic ingredient in VANCOMYCIN is vancomycin hydrochloride. There are twenty-two drug master file entries for this compound. Thirty-one suppliers are listed for this compound. Additional details are available on the vancomycin hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Vancomycin
A generic version of VANCOMYCIN was approved as vancomycin hydrochloride by FRESENIUS KABI USA on March 17th, 1987.
Summary for VANCOMYCIN
| International Patents: | 47 |
| US Patents: | 5 |
| Applicants: | 30 |
| NDAs: | 54 |
| Raw Ingredient (Bulk) Api Vendors: | 37 |
| Clinical Trials: | 423 |
| Patent Applications: | 745 |
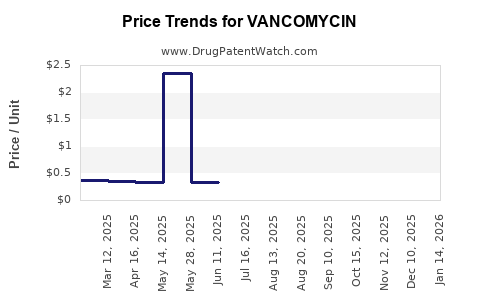
| Drug Prices: | Drug price information for VANCOMYCIN |
| What excipients (inactive ingredients) are in VANCOMYCIN? | VANCOMYCIN excipients list |
| DailyMed Link: | VANCOMYCIN at DailyMed |
See drug prices for VANCOMYCIN
Recent Clinical Trials for VANCOMYCIN
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Universitätsmedizin Mannheim | Phase 2 |
| German Cancer Research Center | Phase 2 |
| Michael Dill | Phase 2 |
Pharmacology for VANCOMYCIN
| Drug Class | Glycopeptide Antibacterial |
Anatomical Therapeutic Chemical (ATC) Classes for VANCOMYCIN
US Patents and Regulatory Information for VANCOMYCIN
VANCOMYCIN is protected by five US patents.
Patents protecting VANCOMYCIN
Glycopeptide compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING BACTERIAL INFECTIONS
Glycopeptide compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING BACTERIAL INFECTIONS
Glycopeptide compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Glycopeptide compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Glycopeptide compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING BACTERIAL INFECTIONS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mylan Labs Ltd | VANCOMYCIN HYDROCHLORIDE | vancomycin hydrochloride | INJECTABLE;INJECTION | 091554-001 | Sep 19, 2011 | AP | RX | No | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Mylan Labs Ltd | VANCOMYCIN HYDROCHLORIDE | vancomycin hydrochloride | POWDER;INTRAVENOUS | 209481-002 | Jul 10, 2018 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Hainan Poly | VANCOMYCIN HYDROCHLORIDE | vancomycin hydrochloride | INJECTABLE;INJECTION | 215821-002 | Nov 18, 2021 | AP | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Fresenius Kabi Usa | VANCOMYCIN HYDROCHLORIDE | vancomycin hydrochloride | INJECTABLE;INJECTION | 062663-003 | Jun 3, 1988 | AP | RX | No | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Xellia Pharms Aps | VANCOMYCIN HYDROCHLORIDE | vancomycin hydrochloride | INJECTABLE;INJECTION | 091377-002 | Sep 9, 2015 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Xellia Pharms Aps | VANCOMYCIN HYDROCHLORIDE | vancomycin hydrochloride | SOLUTION;INTRAVENOUS | 211962-006 | May 13, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Samson Medcl | VANCOMYCIN HYDROCHLORIDE IN PLASTIC CONTAINER | vancomycin hydrochloride | POWDER;INTRAVENOUS | 091532-001 | Jan 6, 2016 | RX | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for VANCOMYCIN
See the table below for patents covering VANCOMYCIN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2852550 | ⤷ Sign Up | |
| South Korea | 102603756 | ⤷ Sign Up | |
| China | 107073072 | 糖肽组合物 (Glycopeptide compositions) | ⤷ Sign Up |
| Saudi Arabia | 517381446 | تركيبات مضاد حيوي (ANTIBIOTIC COMPOSITIONS) | ⤷ Sign Up |
| Mexico | 2017005749 | COMPOSICIONES ANTIBIÓTICAS. (GLYCOPEPTIDE COMPOSITIONS.) | ⤷ Sign Up |
| Japan | 2017533218 | グリコペプチド組成物 | ⤷ Sign Up |
| Brazil | 112017009405 | composições antibióticas. | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |


