TUKYSA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tukysa, and when can generic versions of Tukysa launch?
Tukysa is a drug marketed by Seagen and is included in one NDA. There are seven patents protecting this drug.
This drug has two hundred and seventeen patent family members in forty-five countries.
The generic ingredient in TUKYSA is tucatinib. One supplier is listed for this compound. Additional details are available on the tucatinib profile page.
DrugPatentWatch® Generic Entry Outlook for Tukysa
Tukysa was eligible for patent challenges on April 17, 2024.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 12, 2032. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for TUKYSA
| International Patents: | 217 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 59 |
| Clinical Trials: | 13 |
| Patent Applications: | 124 |
| Drug Prices: | Drug price information for TUKYSA |
| What excipients (inactive ingredients) are in TUKYSA? | TUKYSA excipients list |
| DailyMed Link: | TUKYSA at DailyMed |
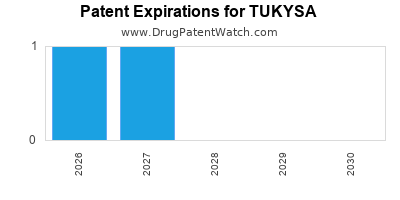

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for TUKYSA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for TUKYSA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| SCRI Development Innovations, LLC | Phase 2 |
| Spanish Breast Cancer Research Group | Phase 2 |
| Criterium, Inc. | Phase 2 |
Pharmacology for TUKYSA
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Cytochrome P450 2C8 Inhibitors Cytochrome P450 3A Inhibitors P-Glycoprotein Inhibitors Tyrosine Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for TUKYSA
US Patents and Regulatory Information for TUKYSA
TUKYSA is protected by seven US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of TUKYSA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting TUKYSA
Treatment of HER2 positive cancers
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMBINATION TREATMENT WITH TUCATINIB AND TRASTUZUMAB OF ADULTS WITH RAS WILD-TYPE HER2-POSITIVE UNRESECTABLE OR METASTATIC COLORECTAL CANCER THAT HAS PROGRESSED FOLLOWING PREVIOUS TREATMENT AS CLAIMED
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF BREAST CANCER INCLUDING HER2 (ERBB2)-POSITIVE OR -OVEREXPRESSING BREAST CANCER
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: COMBINATION TREATMENT WITH TUCATINIB AND TRASTUZUMAB OF ADULTS WITH RAS WILD-TYPE HER2-POSITIVE UNRESECTABLE OR METASTATIC COLORECTAL CANCER THAT HAS PROGRESSED FOLLOWING PREVIOUS TREATMENT AS CLAIMED
Quinazoline analogs as receptor tyrosine kinase inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF BREAST CANCER INCLUDING HER2 (ERBB2)-POSITIVE OR -OVEREXPRESSING BREAST CANCER
N4-phenyl-quinazoline-4-amine derivatives and related compounds as ErbB type I receptor tyrosine kinase inhibitors for the treatment of hyperproliferative diseases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid dispersions of a ERB2 (HER2) inhibitor
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF BREAST CANCER INCLUDING HER2 (ERBB2)-POSITIVE OR -OVEREXPRESSING BREAST CANCER
N4-phenyl-quinazoline-4-amine derivatives and related compounds as ErbB type I receptor tyrosine kinase inhibitors for the treatment of hyperproliferative diseases
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF BREAST CANCER INCLUDING HER2 (ERBB2)-POSITIVE OR -OVEREXPRESSING BREAST CANCER
FDA Regulatory Exclusivity protecting TUKYSA
INDICATED FOR USE IN COMBINATION WITH TRASTUZUMAB AND CAPECITABINE FOR TREATMENT OF ADULT PATIENTS WITH METASTATIC HER2-POSITIVE BREAST CANCER AND BRAIN METASTASES, WHO HAVE RECEIVED ONE OR MORE PRIOR ANTI-HER2-BASED REGIMENS IN THE METASTATIC SETTING
Exclusivity Expiration: ⤷ Sign Up
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Sign Up
TUCATINIB IN COMBINATION WITH TRASTUZUMAB FOR THE TREATMENT OF ADULT PATIENTS WITH RAS WILD-TYPE, HER2-POSITIVE, UNRESECTABLE OR METASTATIC COLORECTAL CANCER THAT HAS PROGRESSED FOLLOWING TREATMENT WITH FLUOROPYRIMIDINE-, OXALIPLATIN-, AND IRINOTECAN-BASED CHEMOTHERAPY
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF ADULT PATIENTS WITH RAS WILD-TYPE, HER2-POSITIVE UNRESECTABLE OR METASTATIC COLORECTAL CANCER THAT HAS PROGRESSED FOLLOWING TREATMENT WITH FLUOROPYRIMIDINE-, OXALIPLATIN-, AND IRINOTECAN-BASED CHEMOTHERAPY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-002 | Apr 17, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-002 | Apr 17, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-002 | Apr 17, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for TUKYSA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Seagen B.V. | Tukysa | tucatinib | EMEA/H/C/005263 Tukysa is indicated in combination with trastuzumab and capecitabine for the treatment of adult patients with HER2‑positive locally advanced or metastatic breast cancer who have received at least 2 prior anti‑HER2 treatment regimens. |
Authorised | no | no | no | 2021-02-11 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for TUKYSA
When does loss-of-exclusivity occur for TUKYSA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 12322039
Estimated Expiration: ⤷ Sign Up
Patent: 17210499
Estimated Expiration: ⤷ Sign Up
Patent: 19200243
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2014009092
Estimated Expiration: ⤷ Sign Up
Patent: 2020010643
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 52058
Estimated Expiration: ⤷ Sign Up
Patent: 14454
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 14000930
Estimated Expiration: ⤷ Sign Up
China
Patent: 3998023
Estimated Expiration: ⤷ Sign Up
Patent: 8498465
Estimated Expiration: ⤷ Sign Up
Patent: 4886853
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 60547
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 140228
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0171578
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 19837
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 65990
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 65990
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 35247
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 2103
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 44514
Estimated Expiration: ⤷ Sign Up
Patent: 14528484
Estimated Expiration: ⤷ Sign Up
Patent: 16027062
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 65990
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 9072
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 3970
Estimated Expiration: ⤷ Sign Up
Patent: 14004551
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 913
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 4942
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 21029
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 65990
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 65990
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 48448
Estimated Expiration: ⤷ Sign Up
Patent: 14119283
Estimated Expiration: ⤷ Sign Up
Patent: 18107710
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 608
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201401459Y
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 65990
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1606123
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 2000312
Estimated Expiration: ⤷ Sign Up
Patent: 140075798
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 50608
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 94769
Estimated Expiration: ⤷ Sign Up
Patent: 22189
Estimated Expiration: ⤷ Sign Up
Patent: 88733
Estimated Expiration: ⤷ Sign Up
Patent: 1330876
Estimated Expiration: ⤷ Sign Up
Patent: 1728323
Estimated Expiration: ⤷ Sign Up
Patent: 2131902
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 1383
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TUKYSA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2090575 | Procédés et produits intermediares pour la preparation de dérivés N4-phényl-quinazoline-4-amine (Processes and intermediates for the preparation of N4-phenyl-quinazoline-4-amine derivatives) | ⤷ Sign Up |
| European Patent Office | 1971601 | DERIVÉS DE LA N4-PHÉNYL-QUINAZOLINE-4-AMINE ET COMPOSÉS SIMILAIRES EN TANT QUE INHIBITEURS DE LA KINASE TYROSINE ERBB TYPE I POUR LE TRAITEMENT DE MALADIES HYPERPROLIFERATIVES (N4-PHENYL-QUINAZOLINE-4 -AMINE DERIVATIVES AND RELATED COMPOUNDS AS ERBB TYPE I RECEPTOR TYROSINE KINASE INHIBITORS FOR THE TREATMENT OF HYPERPROLIFERATIVE DISEASES) | ⤷ Sign Up |
| Poland | 1660090 | ⤷ Sign Up | |
| Taiwan | 201330876 | Solid dispersion | ⤷ Sign Up |
| South Korea | 102490961 | ⤷ Sign Up | |
| Hungary | E035247 | ⤷ Sign Up | |
| Australia | 2019200243 | SOLID DISPERSIONS OF A ERB2 (HER2) INHIBITOR | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TUKYSA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1971601 | 2190026-1 | Sweden | ⤷ Sign Up | PRODUCT NAME: TUCATINIB, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE; NAT. REG. NO/DATE: EU/20/1526 20210212; FIRST REG.: CH 67798 20200507 |
| 1971601 | 132021000000128 | Italy | ⤷ Sign Up | PRODUCT NAME: TUCATINIB OPZIONALMENTE NELLA FORMA DI UN SALE O SOLVATO FARMACEUTICAMENTE ACCETTABILE(TUKYSA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1526, 20210212 |
| 1971601 | PA2021516 | Lithuania | ⤷ Sign Up | PRODUCT NAME: TUKATINIBAS, PASIRINKTINAI FARMACISKAI PRIIMTINOS DRUSKOS ARBA SOLVATO PAVIDALU; REGISTRATION NO/DATE: EU/1/20/1526 20210211 |
| 1971601 | 27/2021 | Austria | ⤷ Sign Up | PRODUCT NAME: TUCATINIB; REGISTRATION NO/DATE: EU/1/20/1526 (MITTEILUNG) 20210212 |
| 1971601 | C 2021 022 | Romania | ⤷ Sign Up | PRODUCT NAME: TUCATINIB OPTIONAL SUB FORMA DE SARE ACCEPTABILA FARMACEUTIC SAU SOLVAT; NATIONAL AUTHORISATION NUMBER: EU/1/20/1526; DATE OF NATIONAL AUTHORISATION: 20210211; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1526; DATE OF FIRST AUTHORISATION IN EEA: 20210211 |
| 1971601 | CR 2021 00025 | Denmark | ⤷ Sign Up | PRODUCT NAME: TUCATINIB, EVENTUELT I FORM AF ET FARMACEUTISK ACCEPTABELT SALT ELLER SOLVAT DERAF; REG. NO/DATE: EU/1/20/1526 20210212 |
| 1971601 | C20210018 00331 | Estonia | ⤷ Sign Up | PRODUCT NAME: TUKATINIIB;REG NO/DATE: EU/1/20/1526 12.02.2021 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


