TRADJENTA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Tradjenta, and what generic alternatives are available?
Tradjenta is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are nine patents protecting this drug and one Paragraph IV challenge.
This drug has four hundred and fifty-nine patent family members in forty-five countries.
The generic ingredient in TRADJENTA is linagliptin. There are nineteen drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the linagliptin profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Tradjenta
A generic version of TRADJENTA was approved as linagliptin by SUNSHINE on August 31st, 2021.
Summary for TRADJENTA
| International Patents: | 459 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 3 |
| Raw Ingredient (Bulk) Api Vendors: | 93 |
| Clinical Trials: | 10 |
| Patent Applications: | 1,898 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for TRADJENTA |
| Drug Sales Revenues: | Drug sales revenues for TRADJENTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRADJENTA |
| What excipients (inactive ingredients) are in TRADJENTA? | TRADJENTA excipients list |
| DailyMed Link: | TRADJENTA at DailyMed |
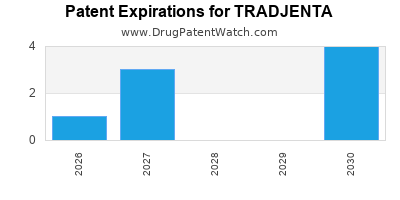
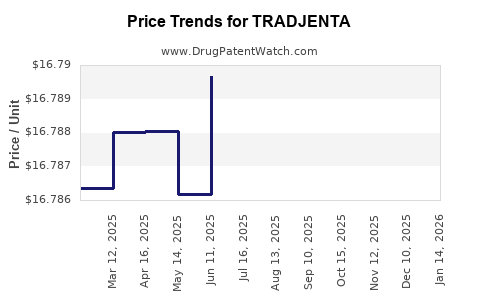
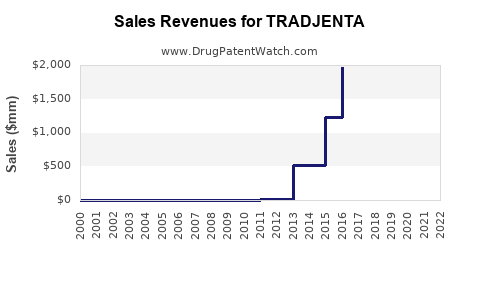
Recent Clinical Trials for TRADJENTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| EMS | Phase 3 |
| University of Miami | Phase 4 |
| Northwell Health | Phase 4 |
Pharmacology for TRADJENTA
| Drug Class | Dipeptidyl Peptidase 4 Inhibitor |
| Mechanism of Action | Dipeptidyl Peptidase 4 Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for TRADJENTA
Paragraph IV (Patent) Challenges for TRADJENTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRADJENTA | Tablets | linagliptin | 5 mg | 201280 | 11 | 2015-05-04 |
US Patents and Regulatory Information for TRADJENTA
TRADJENTA is protected by nine US patents and two FDA Regulatory Exclusivities.
Patents protecting TRADJENTA
Treatment for diabetes in patients inappropriate for metformin therapy
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
DPP IV inhibitor formulations
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: LINAGLIPTIN (5 MG DAILY DOSE) AND METFORMIN (WITH OR WITHOUT INSULIN) FOR TREATING TYPE 2 DIABETES PATIENTS WITH RENAL IMPAIRMENT AND INSUFFICIENT GLYCEMIC CONTROL DESPITE PREVIOUS TREATMENT WITH METFORMIN ALONE OR IN COMBINATION WITH INSULIN
8-[3-amino-piperidin-1-yl]-xanthines, the preparation thereof and their use as pharmaceutical compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Uses of DPP-IV inhibitors
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Treatment for diabetes in patients with inadequate glycemic control despite metformin therapy comprising a DPP-IV inhibitor
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Treatment for diabetes in patients inappropriate for metformin therapy
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Process for the preparation of chiral 8-(3-aminopiperidin-1-yl)-xanthines
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Treatment for diabetes in patients inappropriate for metformin therapy
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting TRADJENTA
REVISIONS TO THE PEDIATRIC USE SUBSECTION OF LABELING TO INCLUDE THE RESULTS FROM CLINICAL STUDY 1218-0091, CONDUCTED TO FULFILL A PEDIATRIC WRITTEN REQUEST
Exclusivity Expiration: ⤷ Sign Up
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRADJENTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| Boehringer Ingelheim | TRADJENTA | linagliptin | TABLET;ORAL | 201280-001 | May 2, 2011 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for TRADJENTA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim International GmbH | Trajenta | linagliptin | EMEA/H/C/002110 Trajenta is indicated in the treatment of type 2 diabetes mellitus to improve glycaemic control in adults:as monotherapyin patients inadequately controlled by diet and exercise alone and for whom metformin is inappropriate due to intolerance, or contraindicated due to renal impairment.as combination therapyin combination with metformin when diet and exercise plus metformin alone do not provide adequate glycaemic control.in combination with a sulphonylurea and metformin when diet and exercise plus dual therapy with these medicinal products do not provide adequate glycaemic control.in combination with insulin with or without metformin, when this regimen alone, with diet and exercise, does not provide adequate glycaemic control. |
Authorised | no | no | no | 2011-08-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for TRADJENTA
When does loss-of-exclusivity occur for TRADJENTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0755
Estimated Expiration: ⤷ Sign Up
Patent: 9930
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 07247193
Estimated Expiration: ⤷ Sign Up
Austria
Patent: 80228
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0711179
Estimated Expiration: ⤷ Sign Up
Patent: 0722388
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 49922
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 12002521
Estimated Expiration: ⤷ Sign Up
Patent: 12002522
Estimated Expiration: ⤷ Sign Up
China
Patent: 1437493
Estimated Expiration: ⤷ Sign Up
Patent: 2526737
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0100507
Estimated Expiration: ⤷ Sign Up
Patent: 0150003
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 11354
Estimated Expiration: ⤷ Sign Up
Patent: 16064
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 23902
Estimated Expiration: ⤷ Sign Up
Patent: 77509
Estimated Expiration: ⤷ Sign Up
Patent: 83819
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 088800
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 6559
Estimated Expiration: ⤷ Sign Up
Patent: 9890
Estimated Expiration: ⤷ Sign Up
Patent: 0802184
Estimated Expiration: ⤷ Sign Up
Patent: 1100958
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 52108
Estimated Expiration: ⤷ Sign Up
Patent: 23902
Estimated Expiration: ⤷ Sign Up
Patent: 77509
Estimated Expiration: ⤷ Sign Up
Patent: 83819
Estimated Expiration: ⤷ Sign Up
Patent: 10241
Estimated Expiration: ⤷ Sign Up
Germany
Patent: 2007009091
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 30442
Estimated Expiration: ⤷ Sign Up
Patent: 72549
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 25210
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 5030
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 78244
Estimated Expiration: ⤷ Sign Up
Patent: 00998
Estimated Expiration: ⤷ Sign Up
Patent: 64720
Estimated Expiration: ⤷ Sign Up
Patent: 87908
Estimated Expiration: ⤷ Sign Up
Patent: 84711
Estimated Expiration: ⤷ Sign Up
Patent: 09535376
Estimated Expiration: ⤷ Sign Up
Patent: 12072187
Estimated Expiration: ⤷ Sign Up
Patent: 13227338
Estimated Expiration: ⤷ Sign Up
Patent: 16104811
Estimated Expiration: ⤷ Sign Up
Patent: 18021082
Estimated Expiration: ⤷ Sign Up
Patent: 20079316
Estimated Expiration: ⤷ Sign Up
Patent: 22075826
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 6969
Estimated Expiration: ⤷ Sign Up
Patent: 8496
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 8617
Estimated Expiration: ⤷ Sign Up
Patent: 08013958
Estimated Expiration: ⤷ Sign Up
Montenegro
Patent: 170
Estimated Expiration: ⤷ Sign Up
Patent: 941
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 2862
Estimated Expiration: ⤷ Sign Up
Patent: 5983
Estimated Expiration: ⤷ Sign Up
Patent: 3426
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 3067
Estimated Expiration: ⤷ Sign Up
Patent: 084256
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 080698
Estimated Expiration: ⤷ Sign Up
Patent: 110666
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 23902
Estimated Expiration: ⤷ Sign Up
Patent: 77509
Estimated Expiration: ⤷ Sign Up
Patent: 83819
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 23902
Estimated Expiration: ⤷ Sign Up
Patent: 83819
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 466
Estimated Expiration: ⤷ Sign Up
Patent: 570
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 1649
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 23902
Estimated Expiration: ⤷ Sign Up
Patent: 83819
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0808361
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1478983
Estimated Expiration: ⤷ Sign Up
Patent: 1710881
Estimated Expiration: ⤷ Sign Up
Patent: 1855323
Estimated Expiration: ⤷ Sign Up
Patent: 2051281
Estimated Expiration: ⤷ Sign Up
Patent: 090009226
Estimated Expiration: ⤷ Sign Up
Patent: 140063896
Estimated Expiration: ⤷ Sign Up
Patent: 150100957
Estimated Expiration: ⤷ Sign Up
Patent: 160128446
Estimated Expiration: ⤷ Sign Up
Patent: 170141812
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 48576
Estimated Expiration: ⤷ Sign Up
Patent: 27409
Estimated Expiration: ⤷ Sign Up
Patent: 38818
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 74843
Estimated Expiration: ⤷ Sign Up
Patent: 20753
Estimated Expiration: ⤷ Sign Up
Patent: 0812648
Estimated Expiration: ⤷ Sign Up
Patent: 1417844
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 942
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 319
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering TRADJENTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20200013100 | 경구 또는 비경구 당뇨병 치료제에 의한 요법에도 불구하고 혈당 조절이 불충분한 환자에 있어서의 당뇨병 치료 (Treatment for diabetes in patients with insufficient glycemic control despite therapy with an oral or non-oral antidiabetic drug) | ⤷ Sign Up |
| Japan | 2010248252 | METHOD FOR REGULATING GLUCOSE METABOLISM AND REAGENT RELATED THERETO | ⤷ Sign Up |
| Japan | 2012514589 | ⤷ Sign Up | |
| South Korea | 102051281 | ⤷ Sign Up | |
| Cyprus | 1115350 | ⤷ Sign Up | |
| Brazil | 0313648 | 8-[3-amino-piperidin-1-il]-xantinas, a preparação das mesmas e uso das mesmas como medicamento | ⤷ Sign Up |
| Netherlands | 300280 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TRADJENTA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1084705 | C300706 | Netherlands | ⤷ Sign Up | PRODUCT NAME: VILDAGLIPTINE; REGISTRATION NO/DATE: EU/1/07/414/001-017 20070926 |
| 2187879 | CA 2017 00019 | Denmark | ⤷ Sign Up | PRODUCT NAME: KOMBINATION AF EMPAGLIFLOZIN OG LINAGLIPTIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/16/1146/001-018 20161115 |
| 1532149 | 8/2012 | Austria | ⤷ Sign Up | PRODUCT NAME: 8-(3-AMINOPIPERIDIN-1-YL)-7-BUT-2-INYL-3-METHYL-1-(4-METHYLCHINAZOLIN-2-YLMETHYL)-3,7-DIHYDROPURIN-2,6-DION UND DESSEN SALZE, INSBES. LINAGLIPTIN; REGISTRATION NO/DATE: EU/1/11/707/001-011 (MITTEILUNG) 20110830 |
| 1084705 | PA2014041 | Lithuania | ⤷ Sign Up | PRODUCT NAME: SITAGLIPTINUM; REGISTRATION NO/DATE: EU/1/07/383/001-024, 2007 03 21 EU/1/07/382/001-024 20070321 |
| 1532149 | PA2011013,C1532149 | Lithuania | ⤷ Sign Up | PRODUCT NAME: LINAGLIPTINUM; REGISTRATION NO/DATE: EU/1/11/707/001, 2011-08-24 EU/1/11/707/002, 2011-08-24 EU/1/11/707/003, 2011-08-24 EU/1/11/707/004, 2011-08-24 EU/1/11/707/005, 2011-08-24 EU/1/11/707/006, 2011-08-24 EU/1/11/707/007, 2011-08-24 EU/1/11/707/008, 2011-08-24 EU/1/11/707/00 2011082 |
| 1084705 | SPC/GB14/086 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: VILDAGLIPTIN; REGISTERED: UK EU/1/07/414/001-017 20070928 |
| 2187879 | 20/2017 | Austria | ⤷ Sign Up | PRODUCT NAME: KOMBINATION VON EMPAGLIFLOZIN UND LINAGLIPTIN ODER PHARMAZEUTISCH VERTRETBARE SALZE; REGISTRATION NO/DATE: EU/1/16/1146 (MITTEILUNG) 20161115 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


