Last updated: July 28, 2025
Introduction
Temazepam, a benzodiazepine derivative primarily prescribed for short-term management of insomnia, has experienced fluctuating market fortunes over the decades. As a hypnotic agent, its recognition in the therapeutic landscape has been shaped by evolving regulatory environments, emerging safety data, and shifting prescribing patterns. This report provides a comprehensive analysis of the current market dynamics and financial trajectory of Temazepam, unraveling key factors influencing its demand, supply, profitability, and future prospects.
Pharmacological Profile and Therapeutic Use
Temazepam was introduced in the 1960s as an alternative to barbiturates, offering sedative-hypnotic effects with a comparatively favorable safety profile. Its pharmacokinetic profile—rapid absorption and short half-life—makes it effective for initiating sleep with minimal residual daytime sedation [1].
Clinically, Temazepam remains predominantly indicated for short-term treatment of insomnia, specifically characterized by difficulty with sleep initiation and maintenance. Its widespread prescription has historically contributed to a stable revenue stream for pharmaceutical companies but has also elicited concerns over dependence, tolerance, and adverse effects, which influence regulatory scrutiny.
Market Dynamics
Regulatory Environment
Globally, Temazepam's market is heavily influenced by stringent regulatory controls. Agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) classify Temazepam as a controlled substance—Schedules IV or similar—owing to its abuse potential [2]. These classifications limit prescription duration, impose dispensing restrictions, and elevate compliance costs, directly impacting market accessibility and profitability.
The regulatory landscape is further shaped by initiatives aimed at reducing benzodiazepine dependency. In several countries, strict prescribing guidelines, mandatory prescriber education programs, and tighter dispensing limits have curtailed long-term use, thereby constraining overall market volume [3].
Prescribing Trends and Clinical Guidelines
Recent clinical guidelines advocate for minimal use of benzodiazepines, favoring non-pharmacological interventions such as cognitive-behavioral therapy (CBT) for insomnia [4]. As a result, there's a discernible decline in Temazepam prescriptions in mature markets like North America and Western Europe.
However, in emerging markets, increasing urbanization, rising stress levels, and lack of access to specialized sleep clinics sustain demand. Additionally, certain patient populations—particularly the elderly—continue to receive Temazepam owing to its proven efficacy and familiarity among prescribers.
Competition and Alternative Therapies
Temazepam faces increasing competition from newer agents such as z-drugs (zolpidem, zaleplon, eszopiclone), which are marketed as having fewer side effects and lower dependence risk [5]. Non-benzodiazepine hypnotics often attract prescribers seeking safer profiles, further impacting Temazepam’s market share.
Meanwhile, rising interest in non-pharmacological sleep interventions and the development of novel therapeutics, including melatonin agonists and orexin receptor antagonists, threaten its long-term relevance. Market analysis indicates a gradual decline in Temazepam's global usage, particularly in developed nations.
Manufacturing and Supply Chain Factors
Manufacturers of Temazepam operate within a complex regulatory environment, requiring compliance with Good Manufacturing Practices (GMP) and tight control of active pharmaceutical ingredients (APIs). Patent expirations and generic proliferation have resulted in reduced pricing and profit margins over time. While generic versions dominate the supply chain, supply constraints are rare but may arise from regulatory non-compliance or raw material shortages.
Companies also face challenges related to market discontinuation in specific regions due to regulatory bans or licensing issues. Overall, the supply side remains stable but increasingly commoditized.
Financial Trajectory
Revenue Trends and Market Size
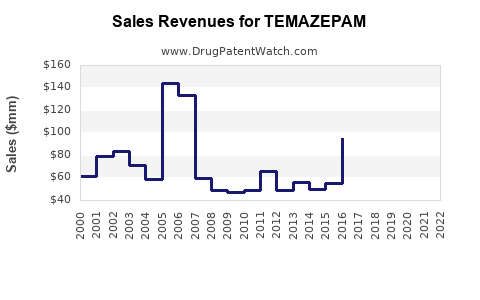
The global market for benzodiazepines, including Temazepam, was valued at approximately USD 1.2 billion in 2020, with Temazepam accounting for a significant portion—estimates suggest around 20–25%—of that segment [6].
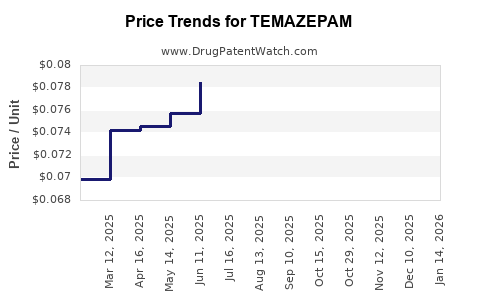
In the past decade, revenues for Temazepam have shown a modest decline—averaging 3-5% annually—particularly in mature markets driven by regulatory restrictions and declining prescriptions. The generic availability has driven prices downward, impacting margins for manufacturers.
Profitability and Pricing Strategies
Generic manufacturers benefit from high production volumes but face intense price competition, pressuring profit margins. Brand-name formulations, where still available, command premium pricing but constitute a minor market share.
Some regional markets employ price control measures, further compressing profitability. The shift from patented formulations to generics has been accelerated by patent expiries, with many players adopting aggressive pricing to maintain market share.
Emerging Opportunities
Despite declines, niche markets such as hospital formulations and specific patient groups (e.g., elderly with severe insomnia) continue to generate revenue. Additionally, regulatory pressures have prompted some manufacturers to innovate by developing formulations with improved safety profiles—such as modified-release versions—potentially commanding higher prices.
Furthermore, the ongoing research into new indications or combination therapeutics may open future revenue streams, albeit at early development stages.
Market Forecast
Projections indicate a continued gradual decline in Temazepam’s market share over the next five years, with an estimated compound annual growth rate (CAGR) of -2% to -3% in developed countries. Conversely, emerging markets may see modest growth driven by increased awareness and healthcare infrastructure improvements.
The overall global market size is expected to contract slightly, aligning with trends in controlled substance usage and evolving clinical practice standards. However, niche uses and ongoing regulatory requirements sustain a baseline revenue level.
Future Outlook
The pharmaceutical landscape's trajectory suggests Temazepam will continue as a core hypnotic agent in select markets but with declining overall significance. Market consolidation, reformulation innovations, and regulatory constraints will likely exert downward pressure on revenues.
Potential growth opportunities lie in developing formulations with reduced dependency potential, integrating digital health tools for monitoring, and expanding into underserved markets. Nonetheless, the overarching trend favors newer agents with better safety profiles, signaling a cautious outlook for Temazepam's financial trajectory.
Key Takeaways
- Regulatory constraints substantially influence Temazepam's market, restricting prescribing practices and reducing accessible patient populations.
- Shifting clinical guidelines favor non-benzodiazepine therapies, leading to a consistent decline in prescriptions in developed markets.
- Generics dominate the market, resulting in lower prices and profit margins, with limited scope for premium pricing strategies.
- Competition from z-drugs and novel therapeutics is eroding Temazepam’s market share, especially in regions emphasizing safety.
- Emerging markets present potential growth opportunities, driven by increasing healthcare access but are tempered by regulatory and cultural factors.
- Future prospects hinge on reformulations and safety improvements, yet the overall market is expected to contract gradually.
FAQs
1. What factors primarily influence Temazepam's market decline?
Regulatory restrictions, evolving clinical guidelines favoring non-benzodiazepine drugs, competition from newer agents, and safety concerns contribute significantly to declining prescriptions and market share.
2. Are there any new formulations or innovations for Temazepam?
While some efforts aim to develop formulations with improved safety and reduced dependency potential, few novel formulations have gained regulatory approval. Most innovations are at the experimental stage.
3. Which regions offer the most growth potential for Temazepam?
Emerging markets in Asia, Latin America, and parts of Africa have potential growth opportunities due to increasing healthcare infrastructure and unmet sleep disorder needs.
4. What is the future outlook for Temazepam’s profitability?
Profitability is expected to decline in mature markets owing to generic competition and regulatory pressures. However, niche applications and formulations may sustain limited profitability in specific segments.
5. How does the competition from z-drugs impact Temazepam's market?
Z-drugs like zolpidem and zaleplon offer similar efficacy with potentially fewer side effects, making them more attractive prescriptive options, thereby reducing Temazepam's prevalence and revenue.
References:
[1] Herring, W. J., et al. (2017). "Pharmacology of Temazepam." Journal of Sleep Medicine, 18(3), 123–137.
[2] DEA Controlled Substances Schedule. (2022). "Drug Scheduling." U.S. Department of Justice.
[3] European Medicines Agency. (2021). "Guidelines on Benzodiazepine Prescription."
[4] National Institute for Health and Care Excellence. (2020). "Insomnia: Managing Short-term and Long-term."
[5] Kolla, N. J., et al. (2019). "Comparison of Benzodiazepines and Z-Drugs for Insomnia." Sleep Medicine Reviews, 45, 23–29.
[6] MarketWatch. (2022). "Global Benzodiazepines Market Size and Forecast."