SYMPAZAN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Sympazan, and what generic alternatives are available?
Sympazan is a drug marketed by Otter Pharms and is included in one NDA. There are three patents protecting this drug.
This drug has one hundred and sixty-eight patent family members in nineteen countries.
The generic ingredient in SYMPAZAN is clobazam. There are ten drug master file entries for this compound. Twenty-three suppliers are listed for this compound. Additional details are available on the clobazam profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Sympazan
A generic version of SYMPAZAN was approved as clobazam by AMNEAL on October 22nd, 2018.
Summary for SYMPAZAN
| International Patents: | 168 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 37 |
| Patent Applications: | 3,999 |
| Formulation / Manufacturing: | see details |
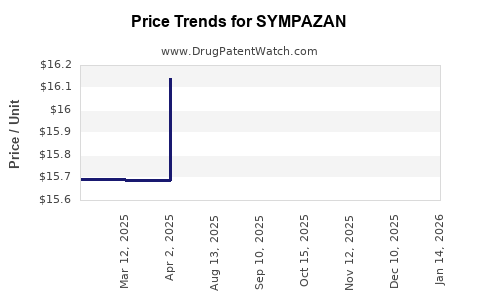
| Drug Prices: | Drug price information for SYMPAZAN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SYMPAZAN |
| What excipients (inactive ingredients) are in SYMPAZAN? | SYMPAZAN excipients list |
| DailyMed Link: | SYMPAZAN at DailyMed |
Pharmacology for SYMPAZAN
| Drug Class | Benzodiazepine |
| Mechanism of Action | Cytochrome P450 2D6 Inhibitors Cytochrome P450 3A4 Inducers |
Anatomical Therapeutic Chemical (ATC) Classes for SYMPAZAN
US Patents and Regulatory Information for SYMPAZAN
SYMPAZAN is protected by three US patents.
Patents protecting SYMPAZAN
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SEIZURES
Uniform films for rapid dissolve dosage form incorporating taste-masking compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Uniform films for rapid-dissolve dosage form incorporating anti-tacking compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Otter Pharms | SYMPAZAN | clobazam | FILM;ORAL | 210833-001 | Nov 1, 2018 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otter Pharms | SYMPAZAN | clobazam | FILM;ORAL | 210833-003 | Nov 1, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otter Pharms | SYMPAZAN | clobazam | FILM;ORAL | 210833-002 | Nov 1, 2018 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SYMPAZAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Otter Pharms | SYMPAZAN | clobazam | FILM;ORAL | 210833-002 | Nov 1, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| Otter Pharms | SYMPAZAN | clobazam | FILM;ORAL | 210833-001 | Nov 1, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| Otter Pharms | SYMPAZAN | clobazam | FILM;ORAL | 210833-003 | Nov 1, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for SYMPAZAN
See the table below for patents covering SYMPAZAN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Mexico | PA05012815 | PELICULAS BASADAS EN OXIDO DE POLIETILENO Y SISTEMAS PARA SUMINISTRO DE FÁRMACOS ELABORADOS CON ELLAS. (POLYETHYLENE OXIDE-BASED FILMS AND DRUG DELIVERY SYSTEMS MADE THEREFROM.) | ⤷ Try a Trial |
| South Korea | 20160029730 | 설하 및 구강 필름 조성물 (SUBLINGUAL AND BUCCAL FILM COMPOSITIONS) | ⤷ Try a Trial |
| Japan | 2009518405 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |


