STEGLUJAN Drug Patent Profile
✉ Email this page to a colleague
When do Steglujan patents expire, and when can generic versions of Steglujan launch?
Steglujan is a drug marketed by Msd Sub Merck and is included in one NDA. There are four patents protecting this drug.
This drug has one hundred and thirty-eight patent family members in fifty-two countries.
The generic ingredient in STEGLUJAN is ertugliflozin; sitagliptin phosphate. One supplier is listed for this compound. Additional details are available on the ertugliflozin; sitagliptin phosphate profile page.
DrugPatentWatch® Generic Entry Outlook for Steglujan
Steglujan was eligible for patent challenges on December 19, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 21, 2030. This may change due to patent challenges or generic licensing.
There have been twenty-nine patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for STEGLUJAN
| International Patents: | 138 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Formulation / Manufacturing: | see details |
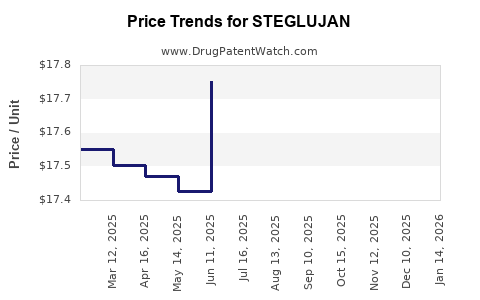
| Drug Prices: | Drug price information for STEGLUJAN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for STEGLUJAN |
| What excipients (inactive ingredients) are in STEGLUJAN? | STEGLUJAN excipients list |
| DailyMed Link: | STEGLUJAN at DailyMed |
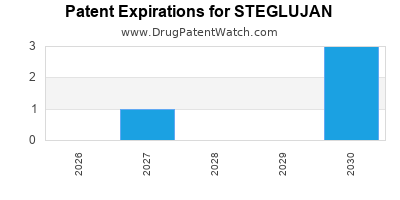
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for STEGLUJAN
Generic Entry Date for STEGLUJAN*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for STEGLUJAN
| Drug Class | Dipeptidyl Peptidase 4 Inhibitor |
| Mechanism of Action | Dipeptidyl Peptidase 4 Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for STEGLUJAN
US Patents and Regulatory Information for STEGLUJAN
STEGLUJAN is protected by four US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of STEGLUJAN is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting STEGLUJAN
Phosphoric acid salt of a dipeptidyl peptidase-IV inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: AN ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES
Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Dioxa-bicyclo[3.2.1]octane-2,3,4-triol derivatives
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: AN ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES
FDA Regulatory Exclusivity protecting STEGLUJAN
REVISION TO THE LABELING TO INCLUDE RESULTS FROM CLINICAL STUDY, MK-8835-004/B1521021, VERTIS CV
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-001 | Dec 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-002 | Dec 19, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-001 | Dec 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-002 | Dec 19, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-001 | Dec 19, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for STEGLUJAN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-001 | Dec 19, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-002 | Dec 19, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-001 | Dec 19, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-002 | Dec 19, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Msd Sub Merck | STEGLUJAN | ertugliflozin; sitagliptin phosphate | TABLET;ORAL | 209805-002 | Dec 19, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for STEGLUJAN
When does loss-of-exclusivity occur for STEGLUJAN?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 99
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 10310956
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 77857
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 120289
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0150107
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 15949
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 96583
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 12011946
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 1983
Estimated Expiration: ⤷ Try a Trial
Patent: 1290267
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 96583
Estimated Expiration: ⤷ Try a Trial
Georgia, Republic of
Patent: 0146104
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 96156
Estimated Expiration: ⤷ Try a Trial
Patent: 13509393
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 016
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 9945
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 96583
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 96583
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 827
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 96583
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1203486
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1426180
Estimated Expiration: ⤷ Try a Trial
Patent: 120093321
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 27179
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 3416
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering STEGLUJAN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Cyprus | 1110784 | ⤷ Try a Trial | |
| Brazil | PI0918841 | derivados de dioxa-biciclo[3.2.1]octano-2,3,4-triol, seus cristais e composições farmacêuticas | ⤷ Try a Trial |
| Jordan | 2230 | مثبطات انزيم داي ببتيديل المحتوي على حلقه غير متجانسه من حلقات البيتا امينو للعلاج او للوقايه من مرض السكري (BETA AMINO HETEROCYCLIC DIPEPTIDYL PEPTIDASE INHIBITORS FOR THE TREATMETN OR PREVENTION OF DIABETES) | ⤷ Try a Trial |
| Costa Rica | 20110077 | DERIVADOS DE DIOXA-BICICLO[3.2.1]OCTANO-2,3,4-TRIOL | ⤷ Try a Trial |
| Eurasian Patent Organization | 200501805 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for STEGLUJAN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1412357 | SPC/GB08/040 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTIN OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, IN PARTICULAR THE MONOPHOSPHATE, PLUS METFORMIN OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, IN PARTICULAR THE HYDROCHLORIDE.; REGISTERED: CH 58450 01-03 20080408; UK EU/1/08/455/001 20080716; UK EU/1/08/455/002 20080716; UK EU/1/08/455/003 20080716; UK EU/1/08/455/004 20080716; UK EU/1/08/455/005 20080716; UK EU/1/08/455/006 20080716; UK EU/1/08/455/007 20080716; UK EU/1/08/455/008 20080716; UK EU/1/08/455/009 20080716; UK EU/1/08/455/010 20080716; UK EU/1/08/455/011 20080716; UK EU/1/08/455/012 20080716; UK EU/1/08/455/013 20080716; UK EU/1/08/455/014 20080716 |
| 2334687 | 132018000000441 | Italy | ⤷ Try a Trial | PRODUCT NAME: ERTUGLIFLOZIN(STEGLATRO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/18/1267, 20180323 |
| 2334687 | 122018000070 | Germany | ⤷ Try a Trial | PRODUCT NAME: ERTUGLIFLOZIN, EINSCHLIESSLICH KRISTALL UMFASSEND ERTUGLIFLOZIN UND ERTUGLIFLOZIN UND L-PYROGLUTAMINSAEURE ALS CO-KRISTALL; REGISTRATION NO/DATE: EU/1/18/1267 20180321 |
| 2334687 | 18C1036 | France | ⤷ Try a Trial | PRODUCT NAME: ERTUGLIFLOZINE,OPTIONNELLEMENT SOUS FORME CRISTALLINE,EN PARTICULIER EN TANT QUE CO-CRISTAL AVEC L'ACIDE L-PYROGLUTANIQUE,ET PARTICULIEREMENT ERTUGLIFOZINE ACIDE L-PYROGLUTANIQUE.; REGISTRATION NO/DATE: EU/1/18/1267 20180323 |
| 1412357 | 42/2007 | Austria | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTIN; REGISTRATION NO/DATE: EU/1/07/383/001-018 (MITTEILUNG) 20070323 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


