SELZENTRY Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Selzentry, and what generic alternatives are available?
Selzentry is a drug marketed by Viiv Hlthcare and is included in two NDAs.
The generic ingredient in SELZENTRY is maraviroc. There are two drug master file entries for this compound. Six suppliers are listed for this compound. Additional details are available on the maraviroc profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Selzentry
A generic version of SELZENTRY was approved as maraviroc by HETERO LABS LTD III on February 7th, 2022.
Summary for SELZENTRY
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 91 |
| Clinical Trials: | 43 |
| Patent Applications: | 5,576 |
| Formulation / Manufacturing: | see details |
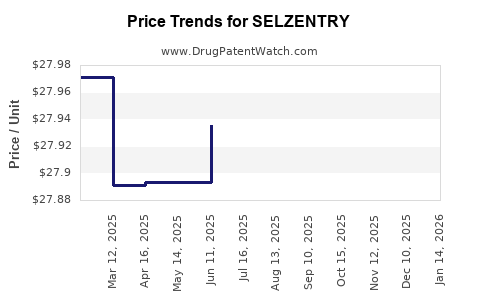
| Drug Prices: | Drug price information for SELZENTRY |
| What excipients (inactive ingredients) are in SELZENTRY? | SELZENTRY excipients list |
| DailyMed Link: | SELZENTRY at DailyMed |
Recent Clinical Trials for SELZENTRY
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hospital Clínic de Barcelona | Phase 2 |
| Hospital Universitario Infanta Leonor | Phase 2 |
| Hospital Clinic of Barcelona | Phase 2 |
Pharmacology for SELZENTRY
| Drug Class | CCR5 Co-receptor Antagonist |
| Mechanism of Action | Chemokine Co-receptor 5 Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for SELZENTRY
US Patents and Regulatory Information for SELZENTRY
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | SELZENTRY | maraviroc | SOLUTION;ORAL | 208984-001 | Nov 4, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-004 | Nov 4, 2016 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Viiv Hlthcare | SELZENTRY | maraviroc | SOLUTION;ORAL | 208984-001 | Nov 4, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-003 | Nov 4, 2016 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-002 | Aug 6, 2007 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-001 | Aug 6, 2007 | AB | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SELZENTRY
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-003 | Nov 4, 2016 | ⤷ Sign Up | ⤷ Sign Up |
| Viiv Hlthcare | SELZENTRY | maraviroc | SOLUTION;ORAL | 208984-001 | Nov 4, 2016 | ⤷ Sign Up | ⤷ Sign Up |
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-002 | Aug 6, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-004 | Nov 4, 2016 | ⤷ Sign Up | ⤷ Sign Up |
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-001 | Aug 6, 2007 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for SELZENTRY
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| ViiV Healthcare B.V. | Celsentri | maraviroc | EMEA/H/C/000811 Celsentri, in combination with other antiretroviral medicinal products, is indicated for treatment experienced adults, adolescents and children of 2 years of age and older and weighing at least 10 kg infected with only CCR5-tropic HIV-1 detectable, |
Authorised | no | no | no | 2007-09-18 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for SELZENTRY
See the table below for patents covering SELZENTRY around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Estonia | 05110 | 3-(3-isoprop l-5-met l-4H-1,2,4-triasool-4- l)ekso-8-asabits klo[3.2.1]oktaani derivaadid, selliste derivaatide valmistamismeetodid, nende valmistamisel kasutatavad vahe hendid, selliseid derivaatesisaldavad kompositsioonid ja kombinatsioonid ning | ⤷ Sign Up |
| Eurasian Patent Organization | 200100589 | ⤷ Sign Up | |
| China | 1150192 | ⤷ Sign Up | |
| Panama | 8488101 | MODULADORES DE CCR5 | ⤷ Sign Up |
| European Patent Office | 1140835 | DERIVES 3,3-BIARYLPIPERIDINE AND 2,2-BIARYLMORPHOLINE (3,3-BIARYLPIPERIDINE AND 2,2-BIARYLMORPHOLINE DERIVATIVES) | ⤷ Sign Up |
| United Kingdom | 0015835 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SELZENTRY
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1284974 | SPC008/2008 | Ireland | ⤷ Sign Up | SPC008/2008: 20081105, EXPIRES: 20220917 |
| 1284974 | C01284974/01 | Switzerland | ⤷ Sign Up | FORMER OWNER: PFIZER INC., US |
| 1284974 | PA2008004,C1284974 | Lithuania | ⤷ Sign Up | PRODUCT NAME: MARAVIROCUM; REGISTRATION NO/DATE: EU/1/07/418/001 - EU/1/07/418/010, 0070918 |
| 1284974 | PA 2008 004, C 1284974 | Lithuania | ⤷ Sign Up | PRODUCT NAME: MARAVIROCUM; REGISTRATION NO/DATE: EU/1/07/418/001 - EU/1/07/418/010, 20070918 |
| 1284974 | 314 | Finland | ⤷ Sign Up | |
| 1284974 | 91417 | Luxembourg | ⤷ Sign Up | 91417, EXPIRES: 20220918 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


