SEASONIQUE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Seasonique, and when can generic versions of Seasonique launch?
Seasonique is a drug marketed by Teva Branded Pharm and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-three patent family members in twenty-two countries.
The generic ingredient in SEASONIQUE is ethinyl estradiol; levonorgestrel. There are twenty-six drug master file entries for this compound. Twenty-eight suppliers are listed for this compound. Additional details are available on the ethinyl estradiol; levonorgestrel profile page.
DrugPatentWatch® Generic Entry Outlook for Seasonique
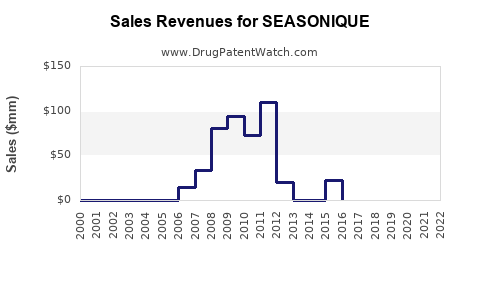
Annual sales in 2021 were $6mm indicating the motivation for generic entry (peak sales were $109mm in 2011).
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (ethinyl estradiol; levonorgestrel), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for SEASONIQUE?
- What are the global sales for SEASONIQUE?
- What is Average Wholesale Price for SEASONIQUE?
Summary for SEASONIQUE
| International Patents: | 63 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 5 |
| Clinical Trials: | 5 |
| Drug Prices: | Drug price information for SEASONIQUE |
| Drug Sales Revenues: | Drug sales revenues for SEASONIQUE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SEASONIQUE |
| What excipients (inactive ingredients) are in SEASONIQUE? | SEASONIQUE excipients list |
| DailyMed Link: | SEASONIQUE at DailyMed |
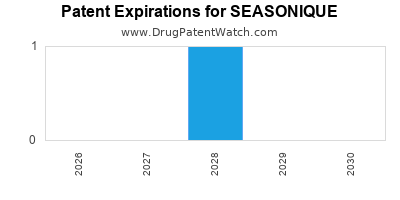

See drug prices for SEASONIQUE
Recent Clinical Trials for SEASONIQUE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Duramed Research | Phase 3 |
| Duramed Research | Phase 2 |
Paragraph IV (Patent) Challenges for SEASONIQUE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SEASONIQUE | Tablets | ethinyl estradiol; levonorgestrel | 0.15 mg/0.03 mg/0.01 mg | 021840 | 1 | 2008-01-22 |
US Patents and Regulatory Information for SEASONIQUE
SEASONIQUE is protected by two US patents.
Patents protecting SEASONIQUE
Oral contraceptives to prevent pregnancy and diminish premenstrual symptomatology
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PREVENTION OF PREGNANCY IN WOMEN WHO ELECT TO USE ORAL CONTRACEPTIVES AS A METHOD OF CONTRACEPTION
Methods of hormonal treatment utilizing contraceptive regimens with continuous estrogen administration
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PREVENTION OF PREGNANCY
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SEASONIQUE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for SEASONIQUE
See the table below for patents covering SEASONIQUE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 2655959 | CONTRACEPTIFS ORAUX POUR EVITER LES GROSSESSES ET DIMINUER LA SYMPTOMATOLOGIE PREMENSTRUELLE (ORAL CONTRACEPTIVES TO PREVENT PREGNANCY AND DIMINISH PREMENSTRUAL SYMPTOMATOLOGY) | ⤷ Sign Up |
| South Africa | 200404249 | ORAL CONTRACEPTIVES TO PREVENT PREGNANCY AND DIMINISH PREMENSTRUAL SYMPTOMATOLOGY | ⤷ Sign Up |
| European Patent Office | 1453521 | CONTRACEPTIFS ORAUX POUR EVITER LES GROSSESSES ET DIMINUER LA SYMPTOMATOLOGIE PREMENSTRUELLE (ORAL CONTRACEPTIVES TO PREVENT PREGNANCY AND DIMINISH PREMENSTRUAL SYMPTOMATOLOGY) | ⤷ Sign Up |
| New Zealand | 591627 | Oral contraceptives to prevent pregnancy and diminish premenstrual symptomatology | ⤷ Sign Up |
| Luxembourg | 93156 | ⤷ Sign Up | |
| Hungary | S1800033 | ⤷ Sign Up | |
| Cyprus | 1114421 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SEASONIQUE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | C20160011 00192 | Estonia | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREEL JA ETUENUEUELOESTRADIOOL;REG NO/DATE: EE 894715 11.11.2015 |
| 1453521 | 2015C/042 | Belgium | ⤷ Sign Up | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), CHANGEMENT DE NOM DU PROPRIETAIRE, NOM/ADRESSE |
| 1453521 | 39/2015 | Austria | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL UND EINE KOMBINATION VON LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 136021 20150224; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 1453521 | 15C0050 | France | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 0136011 | 2000C/027 | Belgium | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 1453521 | 122015000093 | Germany | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 87675.00.00 20150720; FIRST REGISTRATION: SLOWAKEI 17/0017/15-S 20150129 |
| 1453521 | 132016000025143 | Italy | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL ED ETINILESTRADIOLO(SEASONIQUE); AUTHORISATION NUMBER(S) AND DATE(S): 17/0017/15-S, 20150211;042139016, 20150414 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


