SEASONIQUE Drug Patent Profile
✉ Email this page to a colleague
When do Seasonique patents expire, and when can generic versions of Seasonique launch?
Seasonique is a drug marketed by Teva Branded Pharm and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-three patent family members in twenty-two countries.
The generic ingredient in SEASONIQUE is ethinyl estradiol; levonorgestrel. There are twenty-six drug master file entries for this compound. Twenty-six suppliers are listed for this compound. Additional details are available on the ethinyl estradiol; levonorgestrel profile page.
DrugPatentWatch® Generic Entry Outlook for Seasonique
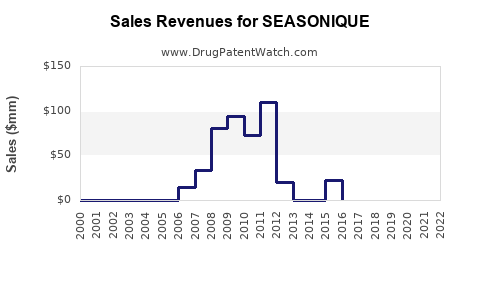
Annual sales in 2021 were $6mm indicating the motivation for generic entry (peak sales were $109mm in 2011).
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are two tentative approvals for the generic drug (ethinyl estradiol; levonorgestrel), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for SEASONIQUE
| International Patents: | 63 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 5 |
| Clinical Trials: | 5 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for SEASONIQUE |
| Drug Sales Revenues: | Drug sales revenues for SEASONIQUE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for SEASONIQUE |
| What excipients (inactive ingredients) are in SEASONIQUE? | SEASONIQUE excipients list |
| DailyMed Link: | SEASONIQUE at DailyMed |
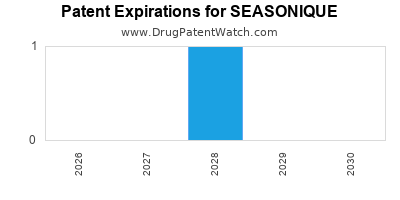

See drug prices for SEASONIQUE
Recent Clinical Trials for SEASONIQUE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Duramed Research | Phase 3 |
| Duramed Research | Phase 2 |
Anatomical Therapeutic Chemical (ATC) Classes for SEASONIQUE
Paragraph IV (Patent) Challenges for SEASONIQUE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SEASONIQUE | Tablets | ethinyl estradiol; levonorgestrel | 0.15 mg/0.03 mg/0.01 mg | 021840 | 2008-01-22 |
US Patents and Regulatory Information for SEASONIQUE
SEASONIQUE is protected by two US patents.
Patents protecting SEASONIQUE
Oral contraceptives to prevent pregnancy and diminish premenstrual symptomatology
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PREVENTION OF PREGNANCY IN WOMEN WHO ELECT TO USE ORAL CONTRACEPTIVES AS A METHOD OF CONTRACEPTION
Methods of hormonal treatment utilizing contraceptive regimens with continuous estrogen administration
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: PREVENTION OF PREGNANCY
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SEASONIQUE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for SEASONIQUE
See the table below for patents covering SEASONIQUE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| New Zealand | 533242 | Oral extended regime contraceptive package | ⤷ Sign Up |
| European Patent Office | 2305230 | Contraceptifs oraux pour empêcher les grossesses (Oral contraceptives to prevent pregnancy) | ⤷ Sign Up |
| Portugal | 1453521 | ⤷ Sign Up | |
| Russian Federation | 2004121155 | ⤷ Sign Up | |
| Hong Kong | 1076716 | ⤷ Sign Up | |
| Australia | 2010201022 | Methods of hormonal treatment utilizing contraceptive regimens with continuous estrogen administration | ⤷ Sign Up |
| Japan | 2006525358 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SEASONIQUE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1214076 | 49/2008 | Austria | ⤷ Sign Up | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON; REGISTRATION NO/DATE: 1-27586 20080612 |
| 1214076 | C01214076/01 | Switzerland | ⤷ Sign Up | PRODUCT NAME: DROSPIRENONE + ETHINYLESTRADIOL; REGISTRATION NUMBER/DATE: SWISSMEDIC 57946 13.06.2008 |
| 1380301 | 2009C/007 | Belgium | ⤷ Sign Up | PRODUCT NAME: DROSPIRENONE-ETHINYLESTRADIOL; AUTHORISATION NUMBER AND DATE: BE321386 20080811 |
| 1214076 | SZ 49/2008 | Austria | ⤷ Sign Up | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON |
| 1453521 | C 2015 029 | Romania | ⤷ Sign Up | PRODUCT NAME: LEVONORGESTREL SI ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: RO 7793/2015/001; DATE OF NATIONAL AUTHORISATION: 20150612; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): SK. 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150129 |
| 0771217 | 07C0001 | France | ⤷ Sign Up | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| 0398460 | C300221 | Netherlands | ⤷ Sign Up | PRODUCT NAME: DROSPIRENON EN ETHINYLESTRADIOL; REGISTRATION NO/DATE: RVG 23827 20000307 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


