SAVAYSA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Savaysa, and what generic alternatives are available?
Savaysa is a drug marketed by Daiichi Sankyo Inc and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and one patent family members in thirty-four countries.
The generic ingredient in SAVAYSA is edoxaban tosylate. There are four drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the edoxaban tosylate profile page.
DrugPatentWatch® Generic Entry Outlook for Savaysa
Savaysa was eligible for patent challenges on January 8, 2019.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 28, 2028. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for SAVAYSA
| International Patents: | 101 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 52 |
| Clinical Trials: | 11 |
| Patent Applications: | 417 |
| Formulation / Manufacturing: | see details |
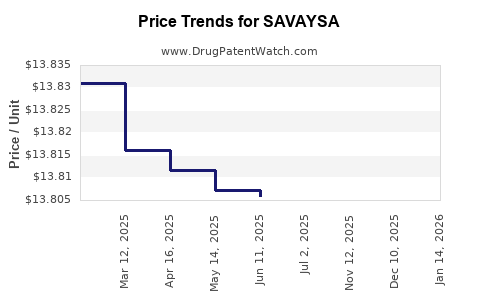
| Drug Prices: | Drug price information for SAVAYSA |
| What excipients (inactive ingredients) are in SAVAYSA? | SAVAYSA excipients list |
| DailyMed Link: | SAVAYSA at DailyMed |
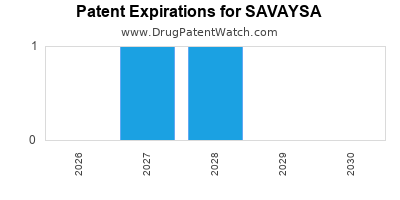
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for SAVAYSA
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for SAVAYSA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Population Health Research Institute | Phase 3 |
| Azienda Ospedaliera di Perugia | Phase 3 |
| Hull University Teaching Hospitals NHS Trust | Phase 3 |
Pharmacology for SAVAYSA
| Drug Class | Factor Xa Inhibitor |
| Mechanism of Action | Factor Xa Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for SAVAYSA
Paragraph IV (Patent) Challenges for SAVAYSA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SAVAYSA | Tablets | edoxaban tosylate | 15 mg, 30 mg and 60 mg | 206316 | 1 | 2019-01-28 |
US Patents and Regulatory Information for SAVAYSA
SAVAYSA is protected by two US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of SAVAYSA is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting SAVAYSA
Diamine derivatives
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
FDA Regulatory Exclusivity protecting SAVAYSA
ADDITIONAL CLINICAL TRIAL INFORMATION ADDED TO PEDIATRIC USE SUBSECTION
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-001 | Jan 8, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-003 | Jan 8, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-002 | Jan 8, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-003 | Jan 8, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-001 | Jan 8, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-001 | Jan 8, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Daiichi Sankyo Inc | SAVAYSA | edoxaban tosylate | TABLET;ORAL | 206316-002 | Jan 8, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for SAVAYSA
When does loss-of-exclusivity occur for SAVAYSA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 08241982
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0809205
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 80039
Estimated Expiration: ⤷ Sign Up
China
Patent: 1652139
Estimated Expiration: ⤷ Sign Up
Patent: 4324015
Estimated Expiration: ⤷ Sign Up
Colombia
Patent: 20955
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0171384
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 19377
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 40867
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 40867
Estimated Expiration: ⤷ Sign Up
Finland
Patent: 40867
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 35990
Estimated Expiration: ⤷ Sign Up
Patent: 800007
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 0790
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 2008129846
Estimated Expiration: ⤷ Sign Up
Patent: 63875
Estimated Expiration: ⤷ Sign Up
Patent: 10090168
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 140867
Estimated Expiration: ⤷ Sign Up
Patent: 2018005
Estimated Expiration: ⤷ Sign Up
Patent: 40867
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 9356
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 1987
Estimated Expiration: ⤷ Sign Up
Patent: 09010474
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 9725
Estimated Expiration: ⤷ Sign Up
Patent: 7109
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 093008
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 012500410
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 40867
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 40867
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 70637
Estimated Expiration: ⤷ Sign Up
Patent: 09139917
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 9497
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 40867
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0906182
Estimated Expiration: ⤷ Sign Up
Patent: 1005906
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1424843
Estimated Expiration: ⤷ Sign Up
Patent: 090122950
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 44803
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 09064
Estimated Expiration: ⤷ Sign Up
Patent: 0845972
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SAVAYSA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Poland | 2140867 | ⤷ Sign Up | |
| Poland | 367532 | ⤷ Sign Up | |
| Hungary | E035990 | ⤷ Sign Up | |
| Norway | 327003 | ⤷ Sign Up | |
| Japan | 4128138 | ⤷ Sign Up | |
| South Africa | 200906182 | PHARMACEUTICAL COMPOSITION | ⤷ Sign Up |
| Brazil | 0210541 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SAVAYSA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1405852 | 2015/045 | Ireland | ⤷ Sign Up | PRODUCT NAME: EDOXABAN, A SALT THEREOF, A SOLVATIC THEREOF, OR AN N-OXIDE THEREOF, IN PARTICULAR EDOXABAN TOSYLATE; REGISTRATION NO/DATE: EU/1/15/993/001-028 20150619 |
| 1405852 | 15C0068 | France | ⤷ Sign Up | PRODUCT NAME: EDOXABAN,UN SEL,SOLVATE OU N-OXYDE DE CELUI-CI,EN PARTICULIER L'EDOXABAN TOSYLATE; REGISTRATION NO/DATE: EU/1/15/993 20150619 |
| 1405852 | CA 2015 00052 | Denmark | ⤷ Sign Up | PRODUCT NAME: EDOXABAN, HERUNDER EDOXABAN TOSILATE; REG. NO/DATE: EU/1/15/993/001-028 20150623 |
| 1405852 | 1590052-5 | Sweden | ⤷ Sign Up | PRODUCT NAME: EDOXABAN, A SALT THEREOF, A SOLVATE THEREOF, IN PARTICULAR EDOXABAN TOSYLATE.; REG. NO/DATE: EU/1/15/993/001-028 20150623 |
| 2140867 | PA2018005,C2140867 | Lithuania | ⤷ Sign Up | PRODUCT NAME: EDOKSABANAS, JO FARMACINIU POZIURIU PRIIMTINA DRUSKA, ARBA BET KURIO IS JU HIDRATAS, YPAC EDOKSABANO P-TOLUENSULFONATO MONOHIDRATAS; REGISTRATION NO/DATE: EU/1/15/993/001-028 20150619 |
| 2140867 | 292 50001-2018 | Slovakia | ⤷ Sign Up | PRODUCT NAME: EDOXABAN VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/15/993 20150623 |
| 1405852 | 122015000077 | Germany | ⤷ Sign Up | PRODUCT NAME: EDOXABAN, DESSEN SALZ, DESSEN SOLVAT, ODER DESSEN N-OXID, INSBESONDERE EDOXABANTOSILAT; REGISTRATION NO/DATE: EU/1/15/993/001-028 20150619 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


