Last updated: November 19, 2025
Introduction
PRED FORTE, a corticosteroid ophthalmic eye drop, is primarily indicated for the treatment of ocular inflammation and allergic conjunctivitis. Its active ingredient, prednisolone acetate, has established its significance within ophthalmology, offering anti-inflammatory benefits with a variety of clinical applications. As the global pharmaceutical landscape evolves, understanding the market dynamics and financial trajectory surrounding PRED FORTE becomes essential for stakeholders including manufacturers, investors, and healthcare providers.
This report examines the key market drivers, competitive landscape, regulatory factors, and economic trends influencing PRED FORTE’s trajectory, along with forecasting its potential growth over the next five years.
Market Overview
Composition and Indications
PRED FORTE is a potent topical corticosteroid formulated for managing ocular inflammatory conditions. It is commonly prescribed for postoperative inflammation, allergic conjunctivitis, keratitis, and uveitis. Its efficacy and safety profile have solidified its position within ophthalmic therapeutics, especially in acute care settings.
Current Market Position
Manufactured by Alcon, a global leader in ophthalmology, PRED FORTE's market presence is reinforced by its reputation for rapid onset of action and reliable anti-inflammatory effects. The drug’s sales are strongly correlated with the prevalence of ocular inflammatory disorders and the frequency of surgical interventions requiring anti-inflammatory prophylaxis.
Market Dynamics
Drivers
1. Rising Prevalence of Ophthalmic Conditions
Age-related ocular conditions such as conjunctivitis, uveitis, and post-surgical inflammation are increasing globally due to demographic shifts. WHO reports detail a growing burden of eye diseases, especially in aging populations, boosting demand for effective corticosteroid treatments like PRED FORTE (WHO, 2020).
2. Expansion of Eye Surgery Procedures
The surge in cataract surgeries, refractive procedures, and corneal transplants elevates the need for post-operative anti-inflammatory agents. PRED FORTE’s fast action and well-tolerated profile make it the preferred choice in many surgical settings (Alcon, 2022).
3. Advances in Ophthalmic Drug Delivery
Innovations in topical ocular formulations improve drug bioavailability, patient compliance, and treatment outcomes. These technological advancements facilitate increased prescriptions for PRED FORTE and similar corticosteroids, supporting sales growth.
4. Growing Healthcare Accessibility
In developing regions, expanding healthcare infrastructure and increased ophthalmic screening programs contribute to higher diagnosis and treatment rates, indirectly boosting PRED FORTE’s market footprint.
Restraints
1. Availability of Alternatives
The market faces competition from non-steroidal anti-inflammatory drugs (NSAIDs), immunomodulators, and preservative-free formulations. These alternatives may offer fewer side effects or simplified dosing, potentially limiting PRED FORTE's growth in certain segments.
2. Stringent Regulatory Environment
The potential for corticosteroid-associated adverse effects (e.g., elevated intraocular pressure, cataract formation) pressure regulatory agencies to impose restrictions or warnings. Such measures could impact prescribing patterns and sales.
3. Growing Awareness of Side Effects
Clinician and patient awareness regarding corticosteroid side effects may lead to more conservative prescribing habits, especially in cases where risks outweigh benefits, thereby tempering sales growth.
Opportunities
1. New Therapeutic Indications
Research exploring corticosteroids' role in managing emerging ocular conditions, including ocular surface diseases linked with autoimmune disorders, presents avenues for expanding PRED FORTE's usage.
2. Market Penetration in Developing Countries
Enhancing availability and affordability in untapped markets can significantly increase volumes, particularly in regions with rising prevalence of eye diseases.
3. Strategic Collaborations
Partnerships with ophthalmic clinics and surgical centers can facilitate larger volume prescriptions, while R&D collaborations may lead to formulation enhancements with improved safety profiles.
Competitive Landscape
Key Players
While Alcon dominates PRED FORTE’s market, it faces competition from brands like FML (fluorometholone), Maxitrol (neomycin, polymyxin B, dexamethasone), and generic corticosteroid formulations. The competition benefits from cost advantages and alternative delivery formulations.
Innovation and Patents
Patent expirations and formulation innovations influence market share dynamics. Alcon’s strategic patent protections for PRED FORTE help maintain exclusivity, whereas generic manufacturers aim to introduce competing products post-expiry.
Market Entry Barriers
Stringent regulatory requirements and high R&D costs serve as barriers for new entrants, preserving Alcon's infrastructural advantage and therapeutic reputation.
Regulatory and Economic Factors
Regulatory Environment
The approval and commercialization of PRED FORTE are contingent on compliance with regional regulatory standards such as FDA (U.S.), EMA (Europe), and respective local agencies. Approvals are generally based on demonstrated safety and efficacy, with post-market surveillance crucial in maintaining market access.
Pricing and Reimbursement
Pricing strategies for ophthalmic corticosteroids vary globally, often influenced by healthcare reimbursement policies. In markets with government reimbursement schemes, PRED FORTE’s availability and affordability are directly impacted, affecting overall sales and financial outlook.
COVID-19 Pandemic Impact
The pandemic initially disrupted elective eye surgeries, temporarily reducing demand for postoperative anti-inflammatory medications. However, as surgical procedures resume and healthcare systems adapt, market recovery is underway. Additionally, increased focus on eye health in pandemic-related telemedicine services may foster future demand.
Financial Trajectory Forecast
The financial outlook for PRED FORTE hinges on several key factors:
-
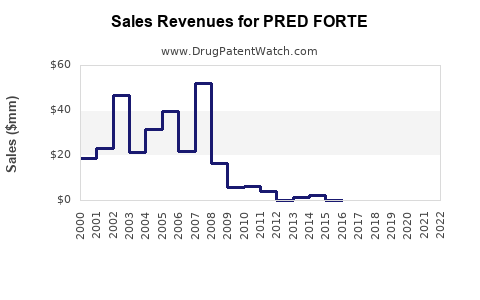
Historical Sales Trends: Over the past decade, PRED FORTE has experienced steady growth due to increased ophthalmic surgeries and aging populations. Data suggests an annual growth rate of approximately 4-6% in established markets.
-
Projected Growth Rate: Considering demographic trends, technological advancements, and expanding ophthalmic procedures, an optimistic projection anticipates a compound annual growth rate (CAGR) of 5-7% over the next five years.
-
Geographical Expansion: Emerging markets, including Asia-Pacific and Latin America, are expected to drive substantial revenue increases, potentially contributing up to 40% of global sales growth.
-
Pricing & Reimbursement Trends: Price adjustments driven by market dynamics and regulatory policies may temper growth slightly but are unlikely to halt upward trends given the drug’s clinical efficacy and positioning.
-
Innovation and New Formulations: Planned investments in new delivery systems and combination drugs could augment demand and expand therapeutic indications, positively impacting revenues.
-
Regulatory Environment: Potential restrictions or stricter safety guidelines could influence sales volume, but proactive compliance and safety monitoring can mitigate adverse effects.
Based on these factors, the future five-year financial trajectory for PRED FORTE is expected to be robust, with potential revenues increasing by approximately 30-35%, contingent on regional expansion and market penetration strategies.
Key Market Challenges and Risks
-
Safety Concerns: Adverse effects such as intraocular pressure elevation could lead to restrictions or decline in usage if not properly managed.
-
Market Saturation: In mature markets, growth potential may plateau unless new indications or formulations are introduced.
-
Competitive Innovation: The advent of safer, non-steroidal alternatives could erode market share.
-
Regulatory Scrutiny: Increased regulatory oversight for corticosteroids may impose additional compliance costs and impact market access.
Conclusion
PRED FORTE’s market dynamics are characterized by steady demand driven by increasing ocular disease prevalence, surgical procedures, and advancements in drug delivery technology. While face competition and regulatory challenges, its core efficacy, established clinical role, and strategic expansion plans underpin a positive financial trajectory. Stakeholders must navigate evolving regulatory and competitive landscapes, leveraging innovation and regional growth opportunities to optimize profitability and market share.
Key Takeaways
- The global growth of ophthalmic surgeries and aging populations will continue to fuel demand for PRED FORTE.
- Competition from NSAIDs and emerging corticosteroid formulations necessitates ongoing innovation and differentiation.
- Expanding presence in emerging markets remains a strategic priority for revenue growth.
- Regulatory and safety considerations will influence prescribing patterns and market access.
- The forecast anticipates a 5-7% CAGR over five years, supporting sustained financial growth.
FAQs
1. What are the primary clinical indications for PRED FORTE?
PRED FORTE is indicated for the treatment of ocular inflammation, allergic conjunctivitis, postoperative inflammation, keratitis, and uveitis.
2. How does PRED FORTE compare to alternative corticosteroid formulations?
PRED FORTE is known for its rapid anti-inflammatory action and high potency, making it suitable for acute conditions. Its formulation minimizes preservative-related side effects, offering a preferred option in many clinical scenarios.
3. What are the key factors affecting PRED FORTE’s market growth?
Market growth is primarily driven by rising ophthalmic surgeries, demographic shifts toward an aging population, technological advances, and expanding healthcare access in emerging markets.
4. Are there any notable patent expirations impacting the market?
Patent protections by Alcon help maintain market exclusivity. Nevertheless, patent expirations of certain formulations may lead to increased generic competition, impacting sales volume and pricing.
5. What future developments could influence PRED FORTE’s financial trajectory?
Introduction of new formulations, expanded indications, strategic partnerships, and market penetration in untapped regions are potential catalysts for growth. Conversely, increased regulatory scrutiny or the emergence of superior alternatives may pose risks.
References
[1] World Health Organization. (2020). Global Data on Vision Loss.
[2] Alcon. (2022). PRED FORTE Product Monograph and Clinical Data.
[3] Industry Reports. (2021). The Ophthalmic Pharmaceutical Market Outlook.
[4] Regulatory Agency Publications. (2022). Ophthalmic Drug Approval Guidelines.