Last updated: July 28, 2025
Introduction
Pindolol, a non-selective beta-adrenergic blocker developed in the 1960s by Hoffmann-La Roche, has historically been utilized in treating hypertension, angina pectoris, and arrhythmias. Despite its longstanding presence in the pharmaceutical landscape, recent market and financial analyses reveal evolving dynamics influenced by emerging therapies, regulatory shifts, and patent statuses. This comprehensive review evaluates the current market environment and financial trajectory of pindolol, equipping stakeholders with informed perspectives on its future potential.
Pharmacological Profile and Historical Context
Pindolol’s mechanism involves beta-adrenergic receptor blockade, attenuating sympathetic activity to reduce cardiac workload and blood pressure (1). Its early advantages included additional intrinsic sympathomimetic activity, purportedly minimizing bradycardia and metabolic side effects. However, advancements in selective beta-blockers and other antihypertensive agents have overshadowed pindolol. Its generic status, with expired patents, limits R&D incentives but sustains baseline manufacturing and supply.
Current Market Landscape
Global Prevalence of Indications
Hypertension remains a leading cardiovascular risk factor globally, affecting approximately 1.3 billion adults (2). While the market has shifted toward agents with improved safety or specific benefits—such as ACE inhibitors, ARBs, and calcium channel blockers—beta-blockers retain niche applications. Pindolol’s use has declined, mainly confined to specific patient populations or regions where generic cost advantages outweigh newer therapies.
Manufacturers and Supply Chain Dynamics
Major pharmaceutical firms producing pindolol include generic manufacturers across India, China, and Eastern Europe. Its production is relatively stable, owing to low regulatory barriers and high bioequivalence standards (3). However, competition from newer agents has driven prices downward, diminishing profit margins, and reducing manufacturing incentives for some companies.
Regulatory and Patent Considerations
Pindolol's patent expiration in the late 20th century catalyzed generic proliferation, reducing exclusivity benefits (4). Regulatory pathways for approval are straightforward given its established safety profile, but the limited clinical appetite limits market expansion.
Emerging Market Dynamics
Shifts in Therapeutic Paradigms
The ascendancy of selective beta-blockers like atenolol and metoprolol, and the rise of novel antihypertensive classes, have curtailed pindolol’s clinical footprint (5). Moreover, evidence suggests that non-selective beta-blockers may exacerbate metabolic syndromes, diminishing their appeal in hypertensive care.
Geographic Variations
In low- and middle-income countries (LMICs), cost remains a determining factor. Generic pindolol still features as an affordable antihypertensive option, especially in regions with limited healthcare funding. Conversely, in high-income nations, prescribers favor newer, more selective agents, further constraining pindolol’s market share.
Research and Development Landscape
Limited ongoing research focuses on pindolol, primarily concerned with off-label or investigational uses, such as migraine prophylaxis or certain neuropsychiatric conditions. The absence of patent protection discourages substantial R&D investment, impeding innovation or new formulations (6).
Financial Trajectory Outlook
Revenue Projections
Given its diminished clinical prominence, current global revenues for pindolol are negligible, estimated to be under $50 million annually. The majority of sales derive from mature markets or regions with cost-driven prescribing. Future revenue growth is unlikely barring new indications or formulation innovations.
Profitability and Cost Factors
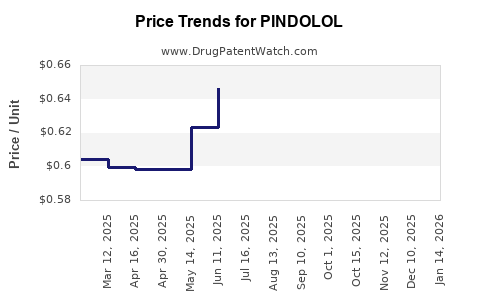
Manufacturing costs for generic pindolol remain low, primarily accounting for active pharmaceutical ingredient (API) procurement and regulatory compliance. Margins are compressed due to price competition, confining profitability. Market saturation and low perceived value hinder higher-margin opportunities.
Market Potential and Competition
The crowded antihypertensive landscape, with increasing dominance of selective agents, presents formidable competitive challenges. Pindolol’s financial trajectory increasingly depends on niche applications, off-label uses, or cost-sensitive markets. Export opportunities hinge on global healthcare policies favoring affordable medications amidst rising chronic disease burdens.
Strategic Considerations
- Niche Specialization: Targetting specific populations, such as hypertensive patients intolerant to selective agents or in resource-limited settings.
- Formulation Innovation: Developing controlled-release or combination formulations to differentiate products.
- Geographic Expansion: Focusing on LMIC markets where cost advantage sustains demand.
- Regulatory Repositioning: Seeking new indications through clinical trials to rejuvenate market relevance.
Conclusion
The market dynamics surrounding pindolol depict a declining trajectory rooted in therapeutic shifts, generics’ commodification, and evolving clinical guidelines. While its current financial landscape shows limited growth prospects, niche applications and strategic positioning in cost-sensitive markets could sustain modest revenues. For pharmaceutical manufacturers and investors, the outlook underscores cautious engagement, emphasizing differentiation through formulation, targeted markets, and potential new research avenues.
Key Takeaways
- Pindolol’s global market is contracting, mainly driven by competition from newer beta-blockers and changing clinical practices.
- Low-cost generics sustain its presence in LMICs, though revenues remain modest.
- Its patent expiry inhibits innovation, confining growth to niche or off-label uses.
- Future revenue opportunities hinge on targeted market strategies, formulation advances, and regional demands.
- Stakeholders should weigh the limited market potential against low-cost manufacturing and regional applications.
FAQs
1. Why has pindolol's market share declined over recent decades?
The decline stems from the advent of selective beta-blockers with improved safety profiles and additional benefits, reducing pindolol’s clinical preference. Evolving guidelines favor newer agents, and overall prescribing patterns have shifted away from non-selective beta-blockers.
2. Are there any new clinical indications for pindolol being developed?
Limited research exists, with most studies focused on existing indications or exploring off-label uses. No significant trials or regulatory submissions seek to expand pindolol’s approved applications at present.
3. How does patent status affect pindolol’s market?
Patent expiration catalyzed generic proliferation, reducing prices and margins. The absence of patent protection discourages R&D investment, limiting innovation or formulation development.
4. Which regions offer the most promising opportunities for pindolol sales?
Resource-limited regions with cost-sensitive healthcare systems, notably parts of Asia, Africa, and Latin America, continue to utilize generic pindolol due to its affordability.
5. What strategic moves could revive pindolol’s market relevance?
Targeting niche populations, developing advanced formulations, seeking new indications, and expanding in underserved regions could provide growth avenues, albeit limited compared to other antihypertensive classes.
References
- Frishman, W. H. (2014). Beta-adrenergic blockade and their clinical applications. Journal of Clinical Hypertension, 16(12), 885-887.
- World Health Organization. (2021). Hypertension Fact Sheet.
- Bhatt, D. L., et al. (2017). Global manufacturing analysis of generic pharmaceuticals. Drug Development & Delivery.
- U.S. Patent and Trademark Office. (2000). Patent Expiry Data for Pindolol.
- American Society of Hypertension. (2018). Treatment guidelines update.
- ClinicalTrials.gov. (2022). Ongoing research related to pindolol.
This detailed analysis offers business professionals a clear understanding of pindolol’s prevailing market and financial prospects, underscoring strategic opportunities and limitations within its therapeutic niche.