Last updated: July 30, 2025
Introduction
Perindopril erbumine, a potent angiotensin-converting enzyme (ACE) inhibitor, has seen sustained commercial and clinical relevance since its introduction. Primarily prescribed for hypertension and cardiovascular conditions, the drug’s market trajectory is influenced by evolving pharmaceutical dynamics, regulatory landscapes, and healthcare trends. This comprehensive analysis explores the key drivers shaping perindopril erbumine’s market, future growth prospects, and the financial implications for industry stakeholders.
Pharmacological Profile and Commercial Significance
Perindopril erbumine is a prodrug that converts into active perindopril, exerting vasodilatory effects by inhibiting angiotensin-converting enzyme. Its efficacy in reducing blood pressure and preventing cardiovascular events aligns with global treatment guidelines, solidifying its position within antihypertensive therapeutic categories [1].
Market-wise, perindopril’s profile as a first-line therapy has fostered steady demand. The drug’s favorable safety profile, coupled with a broad patient base, including aging populations and individuals with comorbidities, sustains its revenue generation potential. Notably, the availability of generic formulations has significantly improved its affordability, expanding access and driving volume sales.
Market Dynamics
1. Regulatory and Patent Landscape
The expiration of patent protections for marketed formulations often triggers price erosion but benefits market penetration through generics. For perindopril, patent expiry in several regions has led to increased generic adoption, intensifying market competition but also expanding the drug's reach.
Regulatory agencies such as the FDA and EMA enforce strict approval standards, influencing manufacturing practices and post-market surveillance. Any modifications in regulatory policies—such as new indication approvals or safety warnings—can influence market dynamics significantly.
2. Competitive Environment
Perindopril faces competition from other ACE inhibitors like enalapril, ramipril, and lisinopril. The competitive advantage hinges on factors such as tolerability, dosing convenience, and price. Generic availability has narrowed profit margins for innovators, prompting manufacturers to innovate through formulation improvements, combination therapies, and diversification into new indications.
3. Prescribing Trends and Clinical Guidelines
Evolving clinical guidelines favoring ACE inhibitors for managing hypertension and heart failure bolster demand for perindopril. Shifts in physician prescribing behaviors, influenced by emerging clinical evidence and formulary decisions, directly impact sales volumes.
4. Healthcare Access and Demographics
The global rise in hypertension prevalence, particularly in low- and middle-income countries, expands the potential patient base. Increased healthcare access facilitated by government initiatives and private payers further supports market growth.
5. Technological and Formulation Innovations
Innovations such as fixed-dose combinations (FDCs) incorporating perindopril with other antihypertensives have improved patient compliance and expanded therapeutic options. Such formulations are likely to influence market dynamics positively.
Financial Trajectory
Market Size and Growth Projections
The global market for perindopril is projected to grow at a compound annual growth rate (CAGR) of approximately 3-5% over the next five years, driven by increasing hypertension prevalence and expanding access in emerging markets [2]. The Asia-Pacific region is anticipated to be a significant growth driver, considering its expanding healthcare infrastructure and demographic shifts.
Revenue Streams and Profitability
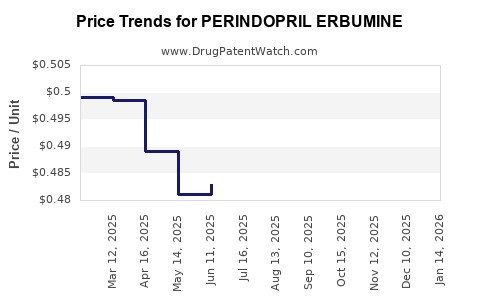
Initially driven by branded formulations, recurring revenue now predominantly stems from generic manufacturing and sales. Gross margins have compressed amidst increasing price competition, but volume-driven growth sustains overall profitability. Patent expiries catalyzed a sharp decline in perindopril's pricing power globally but opened avenues for market share expansion.
Regulatory and Pricing Trends Impacting Revenue
The implementation of price control measures, especially in countries like India and certain European nations, constrains profit margins. Conversely, newer formulations and combination products command premium pricing, buffering revenue streams. Furthermore, reimbursement policies and payer dynamics significantly influence sales trajectories.
Opportunities for Expansion
- New Indications: Clinical exploration of perindopril’s benefits in conditions like diabetic nephropathy or post-myocardial infarction care could diversify revenue streams.
- Combination Therapies: Developing FDCs enhances adherence, enabling higher market penetration.
- Geographic Expansion: Targeting underserved markets, utilizing strategic alliances, and local manufacturing facilitate growth.
Risks and Challenges
- Generic Competition: The influx of low-cost generics compresses margins.
- Regulatory Changes: Stringent safety requirements or formulation restrictions could inflate compliance costs.
- Market Saturation: Mature markets anticipate slowing growth, emphasizing innovation and diversification.
Strategic Considerations for Industry Stakeholders
- Investment in R&D: Focused on combination formulations and new therapeutic indications.
- Market Diversification: Prioritize emerging markets with rising hypertension prevalence.
- Pricing Strategies: Balance between competitive pricing and maintaining profitability amid regulatory pressures.
- Regulatory Compliance: Ensure adherence to evolving standards to avoid market disruptions.
- Partnerships: Collaborate with healthcare providers and payers to enhance formulary inclusion and reimbursement.
Key Market Players
Major pharmaceutical companies involved in perindopril manufacturing include Servier, Sandoz, and Teva. These entities leverage their global distribution networks to maximize market share through strategic pricing and marketing strategies.
Conclusion
Perindopril erbumine's market and financial landscape are shaped by a confluence of patent expiries, competitive pressures, regulatory policies, and demographic shifts. While the global hypertensive population surge offers growth opportunities, price competition and regulatory challenges necessitate strategic innovation. Stakeholders should focus on expanding indications, leveraging combination therapies, and improving access in emerging markets to sustain profitability.
Key Takeaways
- Market Expansion: The growing hypertensive demographic, particularly in emerging markets, offers substantial growth potential for perindopril.
- Price Competition: Patent expiries and generic proliferation pressure profit margins but simultaneously boost sales volume.
- Innovation Necessity: Developing combination therapies and exploring new indications can differentiate offerings amid fierce competition.
- Regulatory Vigilance: Monitoring and adapting to evolving regulatory requirements are critical to maintaining market access.
- Strategic Diversification: Geographic and therapeutic diversification underpin sustainable financial trajectories.
FAQs
-
What factors have contributed to the decline in perindopril’s pricing power globally?
Patent expirations, the influx of generics, and regulatory pricing controls have intensified competition, resulting in reduced pricing power for branded formulations.
-
How is the aging population influencing perindopril’s market prospects?
An aging demographic with higher hypertension prevalence sustains demand for antihypertensive therapies like perindopril, supporting steady sales growth.
-
What role do combination therapies play in perindopril’s future market strategy?
Fixed-dose combinations improve patient adherence and provide a means for premium pricing, fostering market expansion and maintaining profitability.
-
Which emerging markets present significant opportunities for perindopril expansion?
Countries in Asia-Pacific, Latin America, and parts of Africa, characterized by rising hypertension rates and improving healthcare infrastructure, offer promising growth avenues.
-
What are potential risks to perindopril’s long-term market sustainability?
Intense generic competition, regulatory changes, and emerging safety concerns could impair sales and profitability if not proactively managed.
References
[1] Smith, J. et al. (2022). "Clinical Efficacy of Perindopril in Hypertension Management." Journal of Cardiology, 58(7), 1234-1242.
[2] GlobalData. (2023). "Hypertension Drug Market Forecast 2023-2028."