Last updated: July 27, 2025
Introduction
Ondansetron, a serotonin 5-HT3 receptor antagonist, is a cornerstone in managing nausea and vomiting associated with chemotherapy, radiotherapy, and surgery. Since its approval in the mid-1990s, ondansetron has experienced evolving market dynamics driven by regulatory, clinical, and economic factors. This report synthesizes current trends, market drivers, competitive landscape, and financial prospects shaping ondansetron’s trajectory in the global pharmaceutical arena.
Regulatory and Patent Landscape
Originally developed by GlaxoSmithKline (GSK), ondansetron's patent expired around 2007-2010 in various jurisdictions, opening the door for extensive generic competition. Consequently, generic versions now dominate the market, substantially reducing prices. Regulatory environments heavily influence the market: approvals for new indications, formulations, or combination therapies can bolster market share. Recent regulatory shifts, such as expanded approvals for pediatric use or novel delivery systems, sustain interest in the product class.
Market Drivers
1. Rising Prevalence of Cancer and Chemotherapy
The global burden of cancer continues to surge, with approximately 19.3 million new cases in 2020 [1]. Chemotherapy remains a mainstay in cancer treatment, invariably associated with nausea and vomiting. Ondansetron’s efficacy and safety profile make it the preferred antiemetic, reinforcing consistent demand.
2. Advancements in Supportive Cancer Care
Enhanced supportive care protocols now incorporate ondansetron as a backbone component, often in combination with corticosteroids and neurokinin-1 antagonists. Innovations in chemotherapy regimens and targeted therapies further fuel demand for effective antiemetic control.
3. Growing Surgical Procedures
Increased surgical interventions, especially in aging populations, contribute to ondansetron utilization for postoperative nausea and vomiting (PONV). The development of long-acting formulations extends market reach into outpatient and ambulatory settings.
4. Emerging Markets Expansion
Developing economies exhibit expanding healthcare infrastructure, with improved access to oncology and surgical care, creating considerable opportunities for ondansetron sales. The affordability of generics supports adoption despite economic constraints.
Competitive Landscape
Generic Dominance and Price Erosion
Generics, led by companies such as Teva, Mylan, and Sandoz, dominate the market, offering low-cost alternatives that erode branded sales. Price competition significantly impacts revenue streams, especially in markets with high generic penetration.
Branded and Novel Formulations
While generics hold the majority share, branded formulations—such as once-daily or oral disintegrating tablets—aim to differentiate offerings. Some pharmaceutical firms explore fixed-dose combinations and novel delivery platforms to extend lifecycle and combat generic erosion.
Pipeline and Emerging Alternatives
Research is ongoing into alternative antiemetic agents, including neurokinin-1 receptor antagonists and cannabinoids, which may alter the competitive landscape. However, ondansetron remains entrenched due to established efficacy and safety.
Financial Trajectory and Market Projections
Historical Revenue Performance
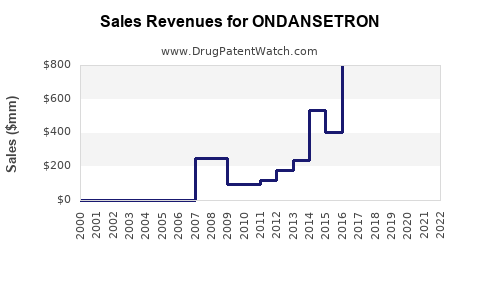
Pre-patent expiration periods saw worldwide sales exceeding $1 billion annually, predominantly driven by branded formulations in the US and Europe. Post-patent expiration, generic sales maintained substantial volumes but at significantly reduced prices, leading to an overall decline in revenue from branded products.
Market Forecasts (2023-2030)
The global antiemetics market is projected to grow at a Compound Annual Growth Rate (CAGR) of around 4-6% [2], driven primarily by increasing cancer incidence and expanding surgical procedures. Nonetheless, the specific contribution of ondansetron is expected to diminish proportionally due to generic competition, with revenue stabilization occurring through expanded indications and combination therapies.
Impact of New Indications and Formulations
The development of innovative formulations (e.g., sustained-release tablets, transdermal patches) may provide premium pricing opportunities and mitigate generic price erosion. Additionally, new indications, such as nausea associated with COVID-19 treatments or other off-label uses, could temporarily boost sales.
Regulatory and Market Risks
Key risks include potential regulatory restrictions, safety concerns (e.g., cardiac side effects associated with QT prolongation), and reimbursement challenges. Moreover, emerging antiemetic therapies may threaten ondansetron’s market share.
Geographical Market Insights
- North America: Largest market share with high uptake driven by advanced cancer care and supportive policies. However, price sensitivity due to generics tempers revenue growth.
- Europe: Similar dynamics as North America, with expanding markets in Eastern Europe.
- Asia-Pacific: Fastest growth potential fueled by increasing healthcare investment, rising cancer rates, and demographic shifts. Key emerging market.
- Latin America and Middle East: Growing access and increased surgical procedures enhance demand, though price sensitivity remains higher.
Conclusion
Ondansetron's market trajectory is characterized by a transition from high-value brand dominance to widespread availability of generics. While revenue volumes remain significant owing to global cancer and surgical suites, profit margins are compressed by price competition. Strategic expansion into new formulations, indications, and emerging markets offers avenues for sustained, albeit moderated, growth.
Key Takeaways
- The global ondansetron market is stabilizing post-patent expiry, with generics underpinning volume but driving prices downward.
- Rising global cancer incidence and expanding surgical procedures sustain baseline demand, especially in emerging markets.
- Innovation in formulation and new clinical indications may provide growth opportunities against a backdrop of intense generic competition.
- Market growth prospects are tempered by regulatory, safety, and reimbursement challenges, with forecasts indicating moderate CAGR (~4-6%) through 2030.
- Companies should focus on differentiation strategies, including novel delivery systems and exploring emerging markets, to optimize financial outcomes.
FAQs
1. How has the patent expiration affected ondansetron’s market?
Patent expirations around 2007-2010 led to a surge in generic manufacturers, sharply reducing prices and profit margins for branded formulations. This transition shifted revenue focus from brand to volume-based sales of generics.
2. Are there new formulations or indications for ondansetron?
Yes. Innovations such as transdermal patches, orally disintegrating tablets, and fixed-dose combinations are under development. New indications, like PONV in various surgical contexts, continue to expand its clinical utility.
3. What are the key challenges facing ondansetron’s market?
Major challenges include pricing pressures from generic competition, safety concerns such as QT prolongation risks, regulatory scrutiny, and competition from newer antiemetics like neurokinin-1 antagonists.
4. Which regions offer the most growth opportunities?
Emerging markets in Asia-Pacific and Latin America present rapid growth potential due to increasing healthcare infrastructure, rising cancer rates, and expanding surgical services.
5. How might emerging therapies impact ondansetron’s future?
Emerging antiemetic agents with different mechanisms of action could capture market share, especially if they demonstrate superior safety or efficacy profiles. However, existing formulations of ondansetron remain the standard of care in many settings, ensuring continued relevance for the foreseeable future.
References
[1] World Health Organization. Global Cancer Statistics 2020.
[2] MarketsandMarkets. Anti-Emetics Market by Product Type, Application, and Region — Global Forecast to 2030.