Last updated: July 27, 2025
Introduction
Olmesartan medoxomil stands as a prominent angiotensin II receptor blocker (ARB), primarily indicated for managing hypertension and reducing cardiovascular risk. Since its approval in the early 2000s, the drug's market trajectory has been shaped by evolving medical guidelines, competitive dynamics, regulatory shifts, and global health trends. This analysis explores the key market drivers, challenges, demographic factors, and financial outlook shaping olmesartan medoxomil’s commercial landscape.
Pharmacological Profile and Therapeutic Position
Olmesartan medoxomil, marketed under brand names such as Benicar, offers a potent antihypertensive effect with once-daily dosing. Its mechanism involves blocking angiotensin II from binding to its receptors, thereby inducing vasodilation and reducing blood pressure. The drug has gained a therapeutic foothold due to its favorable safety profile and once-daily administration convenience.
However, competition among ARBs—including losartan, valsartan, and newer agents—limits market share. The entry of generic formulations has substantially decreased costs, influencing consumption patterns and prescribing behaviors.
Market Dynamics Shaping Olmesartan Medoxomil
1. Regulatory Landscape and Patent Lifespan
Olmesartan's patent expired in numerous jurisdictions around the late 2010s and early 2020s, leading to a surge in generic versions. This patent expiry catalyzed price competition, accelerated generic market penetration, and reduced revenue per prescription for branded formulations[1].
Regulatory environments remain critical; any adverse safety signals, such as reports of gastrointestinal side effects, could influence prescribing policies. Regulatory agencies’ approval of combination therapies (e.g., olmesartan with hydrochlorothiazide) has expanded therapeutic options, influencing market share dynamics.
2. Clinical Guidelines and Prescribing Trends
Major hypertension guidelines (e.g., American College of Cardiology/American Heart Association) increasingly emphasize ARBs as first-line agents, especially in patients intolerant to ACE inhibitors. While olmesartan is recommended, its prescribing rate hinges on clinician familiarity, safety profiles, and patient-specific factors.
Recent guidelines favor combination therapy to achieve better blood pressure control, leading to increased availability of fixed-dose combinations (FDCs). This could dilute demand for monotherapy formulations.
3. Competitive Landscape and Market Penetration
Olmesartan faces stiff competition from other ARBs and emerging antihypertensive classes such as direct renin inhibitors and mineralocorticoid receptor antagonists. Generic proliferation has made inexpensive alternatives widely available.
Pharmaceutical companies are investing in developing next-generation ARBs with improved efficacy or safety profiles. Additionally, biosimilars and fixed-dose combinations are eroding the market share of monotherapy olmesartan formulations.
4. Demographic and Epidemiological Trends
Hypertension remains a pervasive condition globally, with prevalence increasing due to aging populations, urbanization, and lifestyle factors. The World Health Organization estimates over 1.2 billion adults have hypertension worldwide[2].
Developing markets exhibit growing demand for antihypertensives, including olmesartan, driven by expanding healthcare infrastructure and increased awareness. However, affordability and healthcare access limit uptake in some regions.
5. COVID-19 Impact and Market Disruptions
The COVID-19 pandemic introduced temporary disruptions in outpatient services and medication adherence. While hypertension management remained critical, decreased routine care in early pandemic phases suppressed some medication sales. Conversely, increased cardiovascular risk awareness prompts sustained demand for efficacious antihypertensives, including olmesartan.
Financial Trajectory and Revenue Outlook
1. Revenue Trends Pre- and Post-Patent Expiry
Initially, olmesartan achieved robust sales as a branded medication. For instance, in the US, Sanofi reported peak sales exceeding $1 billion annually for Benicar pre-generic entry[3].
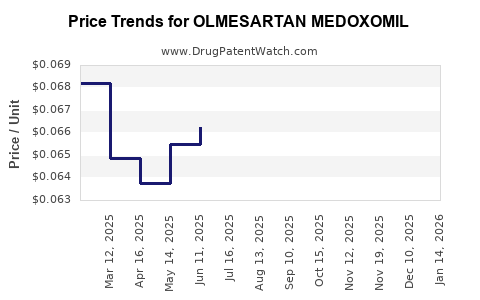
Post-patent expiration, revenue steeply declined due to generic competition. Price erosion averaged 50–70%, depending on the region. Despite volume stability, per-unit revenue diminished, leading to a sharp decline in overall sales for large pharmaceutical firms.
2. Impact of Generics and Biosimilars
The proliferation of generics has introduced intense price competition. Market data indicate a decline in branded olmesartan sales by over 80% within five years of patent expiry[4].
Bioequivalent generics command significantly lower prices, compelling branded product manufacturers to shift focus toward biosimilars and branded combination therapies or to reallocate R&D investments toward novel agents.
3. Market Expansion Through Fixed-Dose Combinations
Fixed-dose combinations (FDCs), such as olmesartan with hydrochlorothiazide, present an opportunity for revenue retention. These formulations enhance patient adherence, broadening market appeal.
The FDC market segment demonstrates higher growth rates, underpinning future revenues, especially in regions emphasizing combination therapy as a standard of care.
4. Regional Market Dynamics
-
United States: Mature market with significant generic penetration and emphasis on combination therapy. Revenue projections for olmesartan are tapering but supplemented by chronic disease management opportunities.
-
Emerging Economies: Higher growth trajectories due to increasing hypertension prevalence and expanding health access. Lower drug prices and licensing enable increased volume sales.
-
European Union: Stringent regulatory climate and cost containment strategies influence sales volume and pricing.
5. Future Financial Outlook
While declining in traditional markets, olmesartan's global sales are expected to stabilize or increase marginally with the growth of combination therapy segment, especially in emerging economies. The potential for biosimilar development could further influence the landscape.
Pharmaceutical companies’ strategic focus may shift toward developing next-generation ARBs or novel antihypertensive agents offering superior efficacy or safety profiles, potentially diminishing olmesartan’s market share.
Market Challenges and Opportunities
-
Challenges:
-
Patent expiry-induced generic competition.
-
Pricing pressures and reimbursement constraints.
-
Market saturation in developed regions.
-
Potential safety concerns impacting prescribing behaviors.
-
Opportunities:
-
Expansion into emerging markets.
-
Development and marketing of fixed-dose combination therapies.
-
Strategic alliances for biosimilar development.
-
Adapting to evolving clinical guidelines favoring personalized medicine.
Conclusion
Olmesartan medoxomil's market has transitioned from a high-revenue branded drug to a commoditized generic product. Its revenue trajectory continues to be influenced by patent expiry, competitive dynamics, demographic shifts, and therapeutic innovations. While its standalone monotherapy sales decline, opportunities lie in combination therapies and growing markets in Asia, Latin America, and Africa.
Pharmaceutical stakeholders must adapt strategies accordingly—focusing on expanding combination formulations, optimizing global distribution, and investing in next-generation agents—to sustain profitability and market relevance.
Key Takeaways
-
Patent expiration significantly impacted olmesartan medoxomil’s revenue, shifting market share toward generic competitors.
-
Generic proliferation has resulted in steep price declines, but increasing demand for combination therapies offers new revenue streams.
-
Demographic trends guarantee a persistent need for antihypertensives, especially in developing countries where hypertension prevalence is rising.
-
Regulatory and guideline shifts influence prescribing patterns, favoring ARBs and combination therapies over monotherapy.
-
Future growth opportunities hinge on innovation, regional market expansion, and strategic formulations, despite intense competition and cost pressures.
Frequently Asked Questions
1. What factors contributed to the decline in revenue for olmesartan medoxomil after patent expiry?
Patent expiry led to the entry of multiple generic versions, resulting in price erosion and increased competition, which decreased revenue for branded olmesartan medoxomil significantly.
2. How do fixed-dose combination therapies impact olmesartan's market share?
FDCs improve patient adherence and are favored in treatment guidelines, providing alternative revenue opportunities and partially offsetting declines in monotherapy sales.
3. Are there any safety concerns associated with olmesartan that influence its market position?
Yes. Reports of gastrointestinal side effects and rare adverse events have led to cautious prescribing, but overall safety profiles remain comparable to other ARBs.
4. What emerging markets present the most growth potential for olmesartan-based therapies?
Regions such as Southeast Asia, Latin America, and Africa exhibit substantial growth potential due to rising hypertension prevalence and expanding healthcare access.
5. How might future innovations affect olmesartan’s market?
Development of newer ARBs with improved efficacy, biosimilars, and personalized medicine approaches could overshadow olmesartan, prompting companies to innovate or shift focus.
References
[1] IMS Health, "Pharmaceutical Market Analysis," 2021.
[2] World Health Organization, "Hypertension Facts," 2022.
[3] Sanofi Annual Report, 2017.
[4] MarketWatch, "Generic ARB Market Trends," 2022.