NEXIUM Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Nexium, and when can generic versions of Nexium launch?
Nexium is a drug marketed by Astrazeneca and Astrazeneca Lp and is included in six NDAs.
The generic ingredient in NEXIUM is esomeprazole sodium. There are seventy-four drug master file entries for this compound. Six suppliers are listed for this compound. Additional details are available on the esomeprazole sodium profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Nexium
A generic version of NEXIUM was approved as esomeprazole sodium by SUN PHARM on March 18th, 2013.
Summary for NEXIUM
| US Patents: | 0 |
| Applicants: | 2 |
| NDAs: | 6 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 44 |
| Clinical Trials: | 160 |
| Patent Applications: | 4,583 |
| Formulation / Manufacturing: | see details |
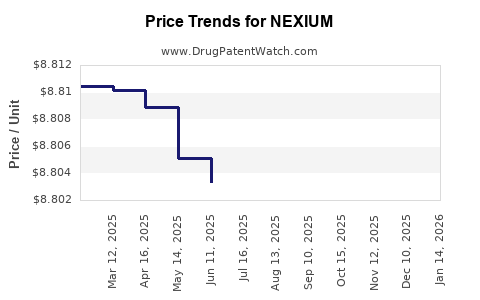
| Drug Prices: | Drug price information for NEXIUM |
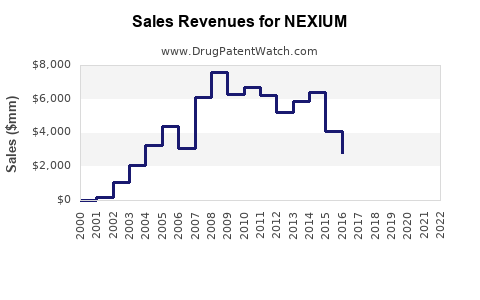
| Drug Sales Revenues: | Drug sales revenues for NEXIUM |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for NEXIUM |
| What excipients (inactive ingredients) are in NEXIUM? | NEXIUM excipients list |
| DailyMed Link: | NEXIUM at DailyMed |
Recent Clinical Trials for NEXIUM
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Nanfang Hospital of Southern Medical University | Phase 4 |
| Liaocheng People's Hospital | Phase 1 |
| CISCRP (Center for Information and Study on Clinical Research Participation) | Phase 3 |
Pharmacology for NEXIUM
| Drug Class | Proton Pump Inhibitor |
| Mechanism of Action | Cytochrome P450 2C19 Inhibitors Proton Pump Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for NEXIUM
Paragraph IV (Patent) Challenges for NEXIUM
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NEXIUM | Delayed-release for Oral Suspension | esomeprazole magnesium | 2.5 mg and 5 mg | 021957 | 1 | 2018-09-24 |
| NEXIUM | Delayed-release for Oral Suspension | esomeprazole magnesium | 10 mg | 022101 | 1 | 2018-07-06 |
| NEXIUM | Delayed-release for Oral Suspension | esomeprazole magnesium | 20 mg and 40 mg | 021957 | 1 | 2013-08-01 |
| NEXIUM | Delayed-release Capsules | esomeprazole magnesium | 20 mg and 40 mg | 021153 | 1 | 2005-08-05 |
US Patents and Regulatory Information for NEXIUM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | NEXIUM | esomeprazole magnesium | CAPSULE, DELAYED REL PELLETS;ORAL | 021153-001 | Feb 20, 2001 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Astrazeneca | NEXIUM | esomeprazole magnesium | FOR SUSPENSION, DELAYED RELEASE;ORAL | 021957-002 | Oct 20, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Astrazeneca | NEXIUM | esomeprazole magnesium | FOR SUSPENSION, DELAYED RELEASE;ORAL | 021957-004 | Dec 15, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NEXIUM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca | NEXIUM | esomeprazole magnesium | CAPSULE, DELAYED REL PELLETS;ORAL | 021153-002 | Feb 20, 2001 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | NEXIUM | esomeprazole magnesium | FOR SUSPENSION, DELAYED RELEASE;ORAL | 022101-001 | Feb 27, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca | NEXIUM | esomeprazole magnesium | FOR SUSPENSION, DELAYED RELEASE;ORAL | 021957-002 | Oct 20, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for NEXIUM
See the table below for patents covering NEXIUM around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 151802 | ⤷ Try a Trial | |
| U.S.S.R. | 873879 | METHOD OF PREPARING BENZIMIDAZOLE DERIVATIVES OR THEIR SALTS | ⤷ Try a Trial |
| Algeria | 1792 | Une nouvelle forme de composé. | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for NEXIUM
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1411900 | 2011C/016 | Belgium | ⤷ Try a Trial | PRODUCT NAME: NAPROXENE ET ESOMEPRAZOLE (SOUS LA FORME D'ESOMEPRAZOLE MAGNESIUM TRIHYDRATE); AUTHORISATION NUMBER AND DATE: BE382505 20101214 |
| 0984957 | PA2011005,C0984957 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: NAPROXENUM + ESOMEPRAZOLUM; REGISTRATION NO/DATE: LT/1/10/2302/001-LT/1/10/2302/012 20110126 |
| 0005129 | SPC/GB99/015 | United Kingdom | ⤷ Try a Trial | SPC/GB99/015, EXPIRES: 20021115 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


