NEXAVAR Drug Patent Profile
✉ Email this page to a colleague
When do Nexavar patents expire, and what generic alternatives are available?
Nexavar is a drug marketed by Bayer Hlthcare and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has eighty-nine patent family members in thirty-nine countries.
The generic ingredient in NEXAVAR is sorafenib tosylate. There are thirteen drug master file entries for this compound. Eight suppliers are listed for this compound. Additional details are available on the sorafenib tosylate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Nexavar
A generic version of NEXAVAR was approved as sorafenib tosylate by MYLAN on September 10th, 2020.
Summary for NEXAVAR
| International Patents: | 89 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 152 |
| Clinical Trials: | 300 |
| Patent Applications: | 3,212 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for NEXAVAR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for NEXAVAR |
| What excipients (inactive ingredients) are in NEXAVAR? | NEXAVAR excipients list |
| DailyMed Link: | NEXAVAR at DailyMed |

Recent Clinical Trials for NEXAVAR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Second Xiangya Hospital of Central South University | Phase 2/Phase 3 |
| Guangzhou First People's Hospital | Phase 2/Phase 3 |
| Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University | Phase 2/Phase 3 |
Pharmacology for NEXAVAR
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Protein Kinase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for NEXAVAR
Paragraph IV (Patent) Challenges for NEXAVAR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NEXAVAR | Tablets | sorafenib tosylate | 200 mg | 021923 | 1 | 2014-02-28 |
US Patents and Regulatory Information for NEXAVAR
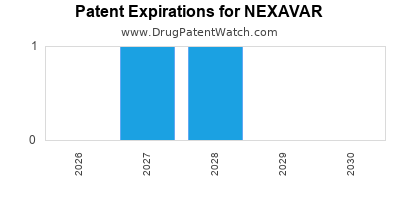
NEXAVAR is protected by four US patents.
Patents protecting NEXAVAR
Thermodynamically stable form of a tosylate salt
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF UNRESECTABLE HEPATOCELLULAR CARCINOMA, ADVANCED RENAL CELL CARCINOMA, OR DIFFERENTIATED THYROID CARCINOMA.
Pharmaceutical composition for the treatment of cancer
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF LOCALLY RECURRENT OR METASTATIC, PROGRESSIVE, DIFFERENTIATED THYROID CARCINOMA REFRACTORY TO RADIOACTIVE IODINE TREATMENT
Pharmaceutical composition for the treatment of cancer
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF ADVANCED RENAL CELL CARCINOMA
Pharmaceutical composition for the treatment of cancer
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF UNRESECTABLE HEPATOCELLULAR CARCINOMA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | NEXAVAR | sorafenib tosylate | TABLET;ORAL | 021923-001 | Dec 20, 2005 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | |
| Bayer Hlthcare | NEXAVAR | sorafenib tosylate | TABLET;ORAL | 021923-001 | Dec 20, 2005 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for NEXAVAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | NEXAVAR | sorafenib tosylate | TABLET;ORAL | 021923-001 | Dec 20, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| Bayer Hlthcare | NEXAVAR | sorafenib tosylate | TABLET;ORAL | 021923-001 | Dec 20, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| Bayer Hlthcare | NEXAVAR | sorafenib tosylate | TABLET;ORAL | 021923-001 | Dec 20, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| Bayer Hlthcare | NEXAVAR | sorafenib tosylate | TABLET;ORAL | 021923-001 | Dec 20, 2005 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for NEXAVAR
When does loss-of-exclusivity occur for NEXAVAR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 4234
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 06222365
Estimated Expiration: ⤷ Sign Up
Austria
Patent: 82693
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 0608840
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 01955
Estimated Expiration: ⤷ Sign Up
China
Patent: 1132779
Estimated Expiration: ⤷ Sign Up
Patent: 4688697
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 48
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0100674
Estimated Expiration: ⤷ Sign Up
Cuba
Patent: 821
Estimated Expiration: ⤷ Sign Up
Patent: 070203
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 11065
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 68579
Estimated Expiration: ⤷ Sign Up
Dominican Republic
Patent: 006000057
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 68579
Estimated Expiration: ⤷ Sign Up
Germany
Patent: 2006017188
Estimated Expiration: ⤷ Sign Up
Guatemala
Patent: 0600096
Estimated Expiration: ⤷ Sign Up
Honduras
Patent: 06009702
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 18019
Estimated Expiration: ⤷ Sign Up
Patent: 09620
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 5517
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 04241
Estimated Expiration: ⤷ Sign Up
Patent: 08531741
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 2319
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 07010856
Estimated Expiration: ⤷ Sign Up
Morocco
Patent: 378
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 1178
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 3834
Estimated Expiration: ⤷ Sign Up
Patent: 075042
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 061345
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 68579
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 68579
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 20283
Estimated Expiration: ⤷ Sign Up
Patent: 07136896
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 0364
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 68579
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 0707638
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1335932
Estimated Expiration: ⤷ Sign Up
Patent: 070111513
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 51612
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 24928
Estimated Expiration: ⤷ Sign Up
Patent: 0700093
Estimated Expiration: ⤷ Sign Up
Tunisia
Patent: 07341
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 673
Estimated Expiration: ⤷ Sign Up
Uruguay
Patent: 410
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering NEXAVAR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Israel | 181734 | פולימורף i של סוראפניב טוזילאט, תכשירי רוקחות המכילים אותו ושימוש בו להכנת תרופות (Polymorph i of sorafenib tosylate, pharmaceutical compositions comprising it and its use in the manufacture of medicaments) | ⤷ Sign Up |
| European Patent Office | 1690853 | Utilisation des diphénylurées à substituants omega-carboxyaryles, inhibitrices de kinase raf (Use of omega-carboxyaryl substituted diphenyl ureas as raf kinase inhibitors) | ⤷ Sign Up |
| Colombia | 5170439 | DIFENIL UREAS SUBSTITUIDAS CON W-CARBOXIARILO Y SUS SALES FARMACEUTICAMENTE ACEPTABLES COMO INHIBIDORES DE RAF QUINASA | ⤷ Sign Up |
| Spain | 2377847 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for NEXAVAR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1140840 | SPC 031/2006 | Ireland | ⤷ Sign Up | SPC 031/2006: 20070528, EXPIRES: 20210718 |
| 1140840 | SPC/GB07/004 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: SORAFENIB AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/06/342/001 20060721 |
| 1140840 | CA 2007 00002 | Denmark | ⤷ Sign Up | PRODUCT NAME: SORAFENIB TOSYLAT |
| 1140840 | SZ 35/2006 | Austria | ⤷ Sign Up | PRODUCT NAME: SORAFENIB UND PHARMAZEUTISCH VERTRAEGLICHE SALZE HIERVON |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


