NEOMYCIN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Neomycin, and what generic alternatives are available?
Neomycin is a drug marketed by Pharmafair, Watson Labs, Xgen Pharms, Bausch And Lomb, Padagis Us, Sciegen Pharms Inc, Alcon Pharms Ltd, Ipharm, Amneal, Sandoz, Saptalis Pharms, Aarxion Anda Hlding, Bristol Myers Squibb, Chartwell Molecular, Lannett, Lilly, Pharmobedient, Roxane, Teva, Fougera, and Pharmaderm. and is included in forty-four NDAs.
The generic ingredient in NEOMYCIN is neomycin sulfate; triamcinolone acetonide. There are nineteen drug master file entries for this compound. Additional details are available on the neomycin sulfate; triamcinolone acetonide profile page.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for NEOMYCIN?
- What are the global sales for NEOMYCIN?
- What is Average Wholesale Price for NEOMYCIN?
Summary for NEOMYCIN
| US Patents: | 0 |
| Applicants: | 21 |
| NDAs: | 44 |
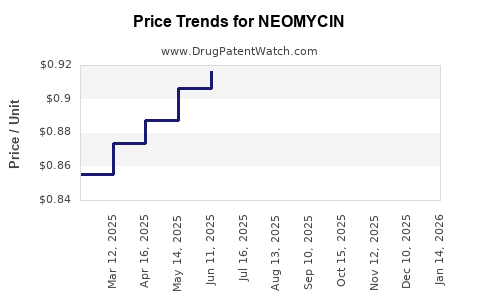
| Drug Prices: | Drug price information for NEOMYCIN |
| DailyMed Link: | NEOMYCIN at DailyMed |