MULTAQ Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Multaq, and what generic alternatives are available?
Multaq is a drug marketed by Sanofi Aventis Us and is included in one NDA. There are three patents protecting this drug and one Paragraph IV challenge.
This drug has fifty-seven patent family members in twenty-eight countries.
The generic ingredient in MULTAQ is dronedarone hydrochloride. There are nineteen drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the dronedarone hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Multaq
A generic version of MULTAQ was approved as dronedarone hydrochloride by LUPIN on January 31st, 2024.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for MULTAQ?
- What are the global sales for MULTAQ?
- What is Average Wholesale Price for MULTAQ?
Summary for MULTAQ
| International Patents: | 57 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 150 |
| Clinical Trials: | 6 |
| Patent Applications: | 1,594 |
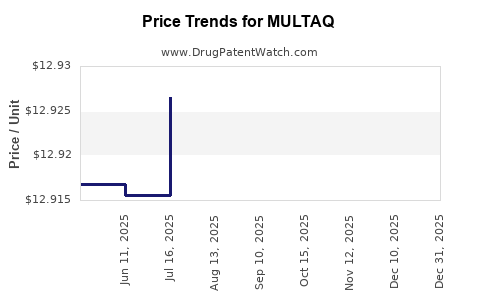
| Drug Prices: | Drug price information for MULTAQ |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for MULTAQ |
| What excipients (inactive ingredients) are in MULTAQ? | MULTAQ excipients list |
| DailyMed Link: | MULTAQ at DailyMed |
Recent Clinical Trials for MULTAQ
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| American Heart Association | Phase 4 |
| Duke Clinical Research Institute | Phase 4 |
| University of Utah | Phase 3 |
Pharmacology for MULTAQ
| Drug Class | Antiarrhythmic |
| Mechanism of Action | Cytochrome P450 2D6 Inhibitors Cytochrome P450 3A Inhibitors P-Glycoprotein Inhibitors |
Paragraph IV (Patent) Challenges for MULTAQ
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| MULTAQ | Tablets | dronedarone hydrochloride | 400 mg | 022425 | 7 | 2013-07-01 |
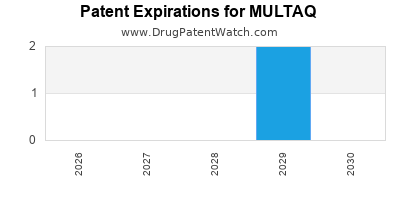
US Patents and Regulatory Information for MULTAQ
MULTAQ is protected by five US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | MULTAQ | dronedarone hydrochloride | TABLET;ORAL | 022425-001 | Jul 1, 2009 | AB | RX | Yes | Yes | 8,410,167 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Sanofi Aventis Us | MULTAQ | dronedarone hydrochloride | TABLET;ORAL | 022425-001 | Jul 1, 2009 | AB | RX | Yes | Yes | 9,107,900 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Sanofi Aventis Us | MULTAQ | dronedarone hydrochloride | TABLET;ORAL | 022425-001 | Jul 1, 2009 | AB | RX | Yes | Yes | 8,602,215 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MULTAQ
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | MULTAQ | dronedarone hydrochloride | TABLET;ORAL | 022425-001 | Jul 1, 2009 | 5,223,510 | ⤷ Get Started Free |
| Sanofi Aventis Us | MULTAQ | dronedarone hydrochloride | TABLET;ORAL | 022425-001 | Jul 1, 2009 | 7,323,493 | ⤷ Get Started Free |
| Sanofi Aventis Us | MULTAQ | dronedarone hydrochloride | TABLET;ORAL | 022425-001 | Jul 1, 2009 | 8,318,800 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for MULTAQ
When does loss-of-exclusivity occur for MULTAQ?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 2950
Estimated Expiration: ⤷ Get Started Free
Patent: 2951
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 09252897
Estimated Expiration: ⤷ Get Started Free
Patent: 09252898
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0910631
Estimated Expiration: ⤷ Get Started Free
Patent: 0911198
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 21489
Estimated Expiration: ⤷ Get Started Free
Patent: 21491
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 09000919
Estimated Expiration: ⤷ Get Started Free
Patent: 09000920
Estimated Expiration: ⤷ Get Started Free
China
Patent: 2065855
Estimated Expiration: ⤷ Get Started Free
Patent: 2065857
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 60064
Estimated Expiration: ⤷ Get Started Free
Patent: 60065
Patent: USO DE DRONEDARONA O UNA SAL ACEPTABLE FARMACEUTICAMENTE DE ESTA PARA LA PREPARACION DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 721
Patent: USO DE DRONEDARONA PARA LA PREPARACIÓN DE UN MEDICAMENTO PARA USO EN LA PREVENCIÓN DE LA HOSPITALIZACIÓN CARDIOVASCULAR O DE LA MORTALIDAD
Estimated Expiration: ⤷ Get Started Free
Patent: 734
Patent: USO DE DRONEDARONA O UNA SAL ACEPTABLE FARMACEUTICAMENTE DE ESTA, PARA LA PREPARACION DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE
Estimated Expiration: ⤷ Get Started Free
Dominican Republic
Patent: 010000299
Patent: USO DE DRONEDARONA PARA LA PREPARACION DE UN MEDICAMENTO PARA USO EN LA PREVENCION DE LA HOSPITALIZACION CARDIOVASCULAR O DE LA MORTALIDAD
Estimated Expiration: ⤷ Get Started Free
Patent: 010000300
Patent: USO DE DRONEDARONA O UNA SAL ACEPTABLE FARMACEUTICAMENTE DE ESTA, PARA LA PREPARACION DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 10010540
Patent: USO DE DRONEDARONA PARA LA PREPARACION DE UN MEDICAMENTO PARA USO EN LA PREVENCION DE LA HOSPITALIZACION CARDIOVASCULAR O DE LA MORTALIDAD
Estimated Expiration: ⤷ Get Started Free
Patent: 10010553
Patent: USO DE DRONEDARONA O UNA SAL ACEPTABLE FARMACEUTICAMENTE DE ESTA, PARA LA PREPARACION DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE
Estimated Expiration: ⤷ Get Started Free
El Salvador
Patent: 10003700
Patent: USO DE DRONEDARONA PARA LA PREPARACION DE UN MEDICAMENTO PARA USO EN LA PREVENCION DE LA HOSPITALIZACION CARDIOVASCULAR O DE LA MORTALIDAD
Estimated Expiration: ⤷ Get Started Free
Patent: 10003701
Patent: USO DE DRONEDARONA O UNA SAL ACEPTABLE FARMACEUTICAMENTE DE ESTA, PARA LA PREPARACION DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 5017
Patent: СПОСОБ ЛЕЧЕНИЯ ПАЦИЕНТОВ С ИСТОРИЕЙ ФИБРИЛЛЯЦИИ ПРЕДСЕРДИЙ ИЛИ ТРЕПЕТАНИЯ ПРЕДСЕРДИЙ, ИЛИ СУЩЕСТВУЮЩИМИ ФИБРИЛЛЯЦИЕЙ ПРЕДСЕРДИЙ ИЛИ ТРЕПЕТАНИЕМ ПРЕДСЕРДИЙ, ПРЕДОТВРАЩАЮЩИЙ ГОСПИТАЛИЗАЦИЮ В КАРДИОЛОГИЧЕСКОЕ ОТДЕЛЕНИЕ (METHOD OF TREATING PATIENTS WITH A HISTORY OF ATRIAL FIBRILLATION OR ATRIAL FLUTTER, OR CURRENT ATRIAL FIBRILLATION OR ATRIAL FLUTTER, PREVENTING CARDIOVASCULAR HOSPITALIZATION)
Estimated Expiration: ⤷ Get Started Free
Patent: 1071204
Patent: ПРИМЕНЕНИЕ ДРОНЕДАРОНА ДЛЯ ПРИГОТОВЛЕНИЯ ЛЕКАРСТВЕННОГО СРЕДСТВА ДЛЯ ПРИМЕНЕНИЯ В ПРЕДОТВРАЩЕНИИ ГОСПИТАЛИЗАЦИИ В КАРДИОЛОГИЧЕСКОЕ ОТДЕЛЕНИЕ ИЛИ СМЕРТНОСТИ
Estimated Expiration: ⤷ Get Started Free
Patent: 1071209
Patent: ПРИМЕНЕНИЕ ДРОНЕДАРОНА ИЛИ ЕГО ФАРМАЦЕВТИЧЕСКИ ПРИЕМЛЕМОЙ СОЛИ ДЛЯ ПОЛУЧЕНИЯ ЛЕКАРСТВЕННОГО СРЕДСТВА ДЛЯ РЕГУЛЯЦИИ УРОВНЯ КАЛИЯ В КРОВИ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 80701
Patent: UTILISATION DE DRONÉDARONE DANS UN MÉDICAMENT UTILISÉ POUR PRÉVENIR UN ÉPISODE CARDIOVASCULAIRE MENANT À L HOSPITALISATION OU À LA MORT (USE OF DRONEDARONE FOR THE PREPARATION OF A MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALIZATION OR OF MORTALITY)
Estimated Expiration: ⤷ Get Started Free
Patent: 80702
Patent: UTILISATION DE LA DRONÉDARONE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI, POUR LA PRÉPARATION D UN MÉDICAMENT PERMETTANT LA RÉGULATION DU NIVEAU DE POTASSIUM DANS LE SANG (USE OF DRONEDARONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, FOR THE PREPARATION OF A MEDICAMENT FOR REGULATING THE POTASSIUM LEVEL IN THE BLOOD)
Estimated Expiration: ⤷ Get Started Free
Patent: 84564
Patent: Utilisation de dronédarone dans un médicament utilisé pour prévenir un épisode cardiovasculaire menant à l’hospitalisation ou prévenir la fibrillation auriculaire (Use of dronedarone for the preparation of a medicament for use in the prevention of cardiovascular hospitalization or in the prevention of atrial fibrillation)
Estimated Expiration: ⤷ Get Started Free
Patent: 95862
Patent: UTILISATION DE LA DRONEDARONE DANS LA PREPARATION D'UN MEDICAMENT UTILISE POUR PREVENIR UN EPISODE CARDIOVASCULAIRE MENANT A L'HOSPITALISATION OU PREVENIR LA FIBRILLATION AURICULAIRE (USE OF DRONEDARONE FOR THE PREPARATION OF A MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALIZATION OR IN THE PREVENTION OF ATRIAL FIBRILLATION)
Estimated Expiration: ⤷ Get Started Free
France
Patent: 30148
Patent: UTILISATION DE LA DRONEDARONE POUR LA PREPARATION D'UN MEDICAMENT DESTINE A LA PREVENTION DE L'HOSPITALISATION CARDIOVASCULAIRE OU DE LA MORTALITE (Use of dronedarone to prepare medicament to prevent mortality and/or cardiovascular hospitalizations in patients having e.g. history of atrial fibrillation/atrial flutter, cerebrovascular accident and non-rheumatic valvular heart disease)
Estimated Expiration: ⤷ Get Started Free
Patent: 30150
Patent: UTILISATION DE LA DRONEDARONE POUR LA PREPARATION D'UN MEDICAMENT DESTINE A REGULER LE TAUX DE POTASSIUM DANS LE SANG
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 11518147
Estimated Expiration: ⤷ Get Started Free
Patent: 11518785
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 3608
Patent: USE OF DRONEDARONE FOR THE PREPARATION OF MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALIZATION OR OF MORTALITY
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 10011400
Patent: USO DE DRONEDARONA O UNA SAL FARMACEUTICAMENTE ACEPTABLE DE LO MISMO, PARA LA PREPARACION DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE. (USE OF DRONEDARONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, FOR THE PREPARATION OF A MEDICAMENT FOR REGULATING THE POTASSIUM LEVEL IN THE BLOOD.)
Estimated Expiration: ⤷ Get Started Free
Patent: 10011414
Patent: USO DE DRONEDARONA PARA LA PREPARACION DE UN MEDICAMENTO PARA USO EN LA PREVENCION DE LA HOSPITALIZACION CARDIOVASCULAR O DE LA MORTALIDAD. (USE OF DRONEDARONE FOR THE PREPARATION OF A MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALIZATION OR OF MORTALITY.)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 354
Patent: استخدام الدرونيدارون في دواء يستخدم لمنع اضطرابات القلب والأوعية الدموية التي قد تؤدي إلى دخول المستشفى أو الوفاة
Estimated Expiration: ⤷ Get Started Free
Patent: 356
Patent: استخدام الدرونيدارون أو ملح مقبول صيدليا ، لإعداد دواء لتنظيم مستوى البوتاسيوم في الدم
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 8623
Patent: Use of a medicament containing dronedarone with food for preventing cardiovascular hospitalization
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1000172
Patent: USO DE DRONEDARONA PARA LA PREPARACIÓN DE UN MEDICAMENTO PARA USO EN LA PREVENCIÓN DE LA HOSPITALIZACIÓN CARDIOVASCULAR O DE LA MORTALIDAD.
Estimated Expiration: ⤷ Get Started Free
Patent: 1000173
Patent: USO DE DRONEDARONA O UNA SAL ACEPTABLE FARMACÉUTICAMENTE DE ÉSTA, PARA LA PREPARACIÓN DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE.
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 091777
Patent: DRONEDARONA O UNA SAL ACEPTABLE FARMACEUTICAMENTE DE LA MISMA
Estimated Expiration: ⤷ Get Started Free
Patent: 091809
Patent: USO DE DRONEDARONA PARA LA PREPARACION DE UN MEDICAMENTO PARA USO EN LA PREVENCION DE LA HOSPITALIZACION CARDIOVASCULAR O DE LA MORTALIDAD
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1007248
Patent: USE OF DRONEDARONE FOR THE PREPARATION OF A MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALIZATION OR OF MORTALITY
Estimated Expiration: ⤷ Get Started Free
Patent: 1007391
Patent: USE OF DRONEDARONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, FOR THE PREPARATION OF A MEDICAMENT FOR REGULATING THE POTASSIUM IN THE BLOOD
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 100135814
Patent: USE OF DRONEDARONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, FOR THE PREPARATION OF A MEDICAMENT FOR REGULATING THE POTASSIUM LEVEL IN THE BLOOD
Estimated Expiration: ⤷ Get Started Free
Patent: 100135909
Patent: USE OF DRONEDARONE FOR THE PREPARATION OF A MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALIZATION OR OF MORTALITY
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 19298
Estimated Expiration: ⤷ Get Started Free
Patent: 0946108
Patent: Use of dronedarone or a pharmaceutically acceptable salt thereof, for the preparation of a medicament for regulating the potassium level in the blood
Estimated Expiration: ⤷ Get Started Free
Patent: 0948354
Patent: Use of dronedarone for the preparation of a medicament for use in the prevention of cardiovascular hospitalization or of mortality
Estimated Expiration: ⤷ Get Started Free
Patent: 1529068
Patent: Use of DRONEDARONE for the preparation of a medicament for use in the prevention of cardiovascular hospitalization or of mortality
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 10000454
Patent: USE OF DRONEDARONE FOR THE PREPARATION OF A MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALISATION OR OF MORTATILY
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 8980
Patent: ЗАСТОСУВАННЯ ДРОНЕДАРОНУ ДЛЯ ПРОФІЛАКТИКИ СЕРЦЕВО-СУДИННИХ ГОСПІТАЛІЗАЦІЙ (USE OF DRONEDARONE FOR THE PREPARATION OF A MEDICAMENT FOR USE IN THE PREVENTION OF CARDIOVASCULAR HOSPITALIZATION OR OF MORTALITY)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 767
Patent: USO DE DRONEDARONA PARA LA PREPARACIÓN DE UN MEDICAMENTO PARA USO EN LA PREVENCIÓN DE LA HOSPITALIZACIÓN CARDIOVASCULAR O DE LA MORTALIDAD
Estimated Expiration: ⤷ Get Started Free
Patent: 768
Patent: USO DE DRONEDARONA O UNA SAL ACEPTABLE FARMACÉUTICAMENTE DE ÉSTA, PARA LA PREPARACIÓN DE UN MEDICAMENTO PARA REGULAR EL NIVEL DE POTASIO EN LA SANGRE
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering MULTAQ around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hungary | 211656 | ⤷ Get Started Free | |
| Argentina | 072951 | ⤷ Get Started Free | |
| Hong Kong | 1000108 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MULTAQ
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1007030 | C01007030/01 | Switzerland | ⤷ Get Started Free | PRODUCT NAME: DRONEDARONUM; REGISTRATION NUMBER/DATE: SWISSMEDIC 59292 16.09.2009 |
| 1007030 | SPC/GB10/029 | United Kingdom | ⤷ Get Started Free | PRODUCT NAME: DRONEDARONE, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; REGISTERED: UK EU/1/09/591/001 20091126; UK EU/1/09/591/002 20091126; UK EU/1/09/591/003 20091126; UK EU/1/09/591/004 20091126 |
| 1007030 | CA 2010 00018 | Denmark | ⤷ Get Started Free | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
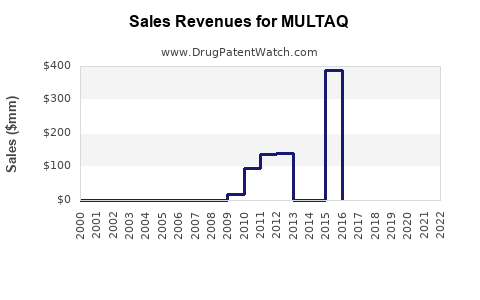
Market Dynamics and Financial Trajectory for MULTAQ (Dronedarone): An In-Depth Analysis
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
