MOBIC Drug Patent Profile
✉ Email this page to a colleague
When do Mobic patents expire, and when can generic versions of Mobic launch?
Mobic is a drug marketed by Boehringer Ingelheim and is included in one NDA.
The generic ingredient in MOBIC is meloxicam. There are twenty-two drug master file entries for this compound. Forty-seven suppliers are listed for this compound. Additional details are available on the meloxicam profile page.
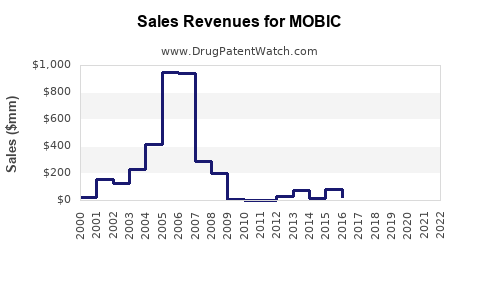
DrugPatentWatch® Litigation and Generic Entry Outlook for Mobic
A generic version of MOBIC was approved as meloxicam by AVONDALE PHARMS on June 1st, 2004.
Summary for MOBIC
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 145 |
| Patent Applications: | 2,932 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for MOBIC |
| What excipients (inactive ingredients) are in MOBIC? | MOBIC excipients list |
| DailyMed Link: | MOBIC at DailyMed |

Anatomical Therapeutic Chemical (ATC) Classes for MOBIC
US Patents and Regulatory Information for MOBIC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | MOBIC | meloxicam | TABLET;ORAL | 020938-001 | Apr 13, 2000 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Boehringer Ingelheim | MOBIC | meloxicam | TABLET;ORAL | 020938-002 | Aug 23, 2000 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for MOBIC
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Norbrook Laboratories (Ireland) Limited | Loxicom | meloxicam | EMEA/V/C/000141 DogsAlleviation of inflammation and pain in both acute and chronic musculoskeletal disorders. To reduce postoperative pain and inflammation following orthopaedic and soft-tissue surgery.CatsAlleviation of inflammation and pain in chronic musculoskeletal disorders in cats. To reduce postoperative pain after ovariohysterectomy and minor soft-tissue surgery.CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle. For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.HorsesFor use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.For the relief of pain associated with equine colic. |
Authorised | yes | no | no | 2009-02-10 | |
| Le Vet Beheer B.V. | Novaquin | meloxicam | EMEA/V/C/003866 Alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders in horses. |
Authorised | no | no | no | 2015-09-08 | |
| Le Vet Beheer B.V | Meloxidolor | meloxicam | EMEA/V/C/002590 DogsAlleviation of inflammation and pain in both acute and chronic musculoskeletal disorders.Reduction of postoperative pain and inflammation following orthopaedic and soft-tissue surgery.CatsReduction of postoperative pain after ovariohysterectomy and minor soft-tissue surgery.CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs.For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.For the relief of postoperative pain associated with minor soft-tissue surgery such as castration.For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.HorsesFor use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders.For the relief of pain associated with equine colic. |
Authorised | yes | no | no | 2013-04-22 | |
| Boehringer Ingelheim Vetmedica GmbH | Novem | meloxicam | EMEA/V/C/000086 Novem 5-mg/ml solution for injection for cattle and pigs:CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For the relief of postoperative pain following dehorning in calves.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.For the relief of postoperative pain associated with minor soft-tissue surgery such as castration.Novem 20-mg/ml solution for injection for cattle and pigs:CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.For the relief of postoperative pain following dehorning in calves.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation.For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.Novem 40 mg/ml solution for injection for cattle:For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs in cattle.For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy. |
Authorised | no | no | no | 2004-03-02 | |
| Boehringer Ingelheim Vetmedica GmbH | Metacam | meloxicam | EMEA/V/C/000033 Cats:Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures, e.g. orthopaedic and soft tissue surgery.Alleviation of pain and inflammation in acute and chronic musculo-skeletal disorders.Reduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.Cattle:For use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs.For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle.For the relief of post-operative pain following dehorning in calves.For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.Dogs:Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders.Reduction of post-operative pain and inflammation following orthopaedic and soft tissue surgery.Horses:For use in the alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders.For the relief of pain associated with equine colic.Alleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders.Pigs: For use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation.For the relief of post-operative pain associated with minor soft tissue surgery such as castration.For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy.Guinea pigs:Alleviation of mild to moderate post-operative pain associated with soft tissues surgery such as male castration. |
Authorised | no | no | no | 1998-01-07 | |
| Chanelle Pharmaceuticals Manufacturing Limited | Rheumocam | meloxicam | EMEA/V/C/000121 DogsAlleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders in dogs. To reduce post-operative pain and inflammation following orthopaedic and soft tissue surgery.CatsReduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.Alleviation of mild to moderate post-operative pain and inflammation following surgical procedures in cats, e.g. orthopaedic and soft tissue surgery.Alleviation of pain and inflammation in acute and chronic musculo-skeletal disorders in cats.CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs. For use in diarrhoea in combination with oral re-hydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.PigsFor use in non-infectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitis-metritis-agalactia syndrome) with appropriate antibiotic therapy. For the relief of post operative pain associated with minor soft tissue such as castration.HorsesAlleviation of inflammation and relief of pain in both acute and chronic musculo-skeletal disorders in horses..For the relief of pain associated with equine colic. |
Authorised | yes | no | no | 2008-01-10 | |
| Emdoka bvba | Emdocam | meloxicam | EMEA/V/C/002283 CattleFor use in acute respiratory infection with appropriate antibiotic therapy to reduce clinical signs. For use in diarrhoea in combination with oral rehydration therapy to reduce clinical signs in calves of over one week of age and young, non-lactating cattle. For adjunctive therapy in the treatment of acute mastitis, in combination with antibiotic therapy.PigsFor use in noninfectious locomotor disorders to reduce the symptoms of lameness and inflammation. For adjunctive therapy in the treatment of puerperal septicaemia and toxaemia (mastitits-metritis-agalactia syndrome) with appropriate antibiotic therapy.HorsesFor use in the alleviation of inflammation and relief of pain in both acute and chronic musculoskeletal disorders. For the relief of pain associated with equine colic.Dogs: Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders. Reduction of post-operative pain and inflammation following orthopaedic and soft tissue surgery.Cats:Reduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery. |
Authorised | yes | no | no | 2011-08-18 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |


