LATISSE Drug Patent Profile
✉ Email this page to a colleague
When do Latisse patents expire, and what generic alternatives are available?
Latisse is a drug marketed by Abbvie and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
This drug has seventeen patent family members in eight countries.
The generic ingredient in LATISSE is bimatoprost. There are twelve drug master file entries for this compound. Twelve suppliers are listed for this compound. Additional details are available on the bimatoprost profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Latisse
A generic version of LATISSE was approved as bimatoprost by APOTEX on December 1st, 2014.
Summary for LATISSE
| International Patents: | 17 |
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 74 |
| Clinical Trials: | 8 |
| Patent Applications: | 4,862 |
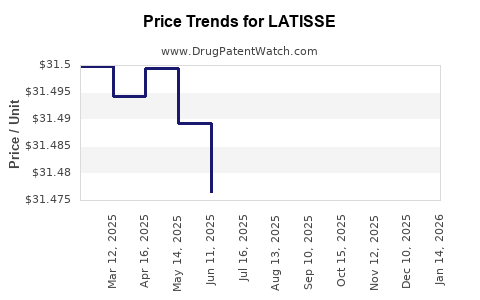
| Drug Prices: | Drug price information for LATISSE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LATISSE |
| What excipients (inactive ingredients) are in LATISSE? | LATISSE excipients list |
| DailyMed Link: | LATISSE at DailyMed |
Recent Clinical Trials for LATISSE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Tulane University | Early Phase 1 |
| Allergan | Early Phase 1 |
| Allergan | Phase 3 |
Pharmacology for LATISSE
| Drug Class | Prostaglandin Analog |
Anatomical Therapeutic Chemical (ATC) Classes for LATISSE
Paragraph IV (Patent) Challenges for LATISSE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LATISSE | Topical Solution | bimatoprost | 0.03% | 022369 | 1 | 2010-05-03 |
US Patents and Regulatory Information for LATISSE
LATISSE is protected by two US patents.
Patents protecting LATISSE
Method of enhancing hair growth
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF STIMULATING HAIR GROWTH
Method of enhancing hair growth
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF INCREASING HAIR GROWTH
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | LATISSE | bimatoprost | SOLUTION/DROPS;TOPICAL | 022369-001 | Dec 24, 2008 | AT | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LATISSE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | LATISSE | bimatoprost | SOLUTION/DROPS;TOPICAL | 022369-001 | Dec 24, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | LATISSE | bimatoprost | SOLUTION/DROPS;TOPICAL | 022369-001 | Dec 24, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | LATISSE | bimatoprost | SOLUTION/DROPS;TOPICAL | 022369-001 | Dec 24, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for LATISSE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AbbVie Deutschland GmbH & Co. KG | Lumigan | bimatoprost | EMEA/H/C/000391 Reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers)., |
Authorised | no | no | no | 2002-03-08 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for LATISSE
See the table below for patents covering LATISSE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2019001828 | 自然に生成しないプロスタグランジンを使用した脱毛症治療用組成物及びその治療方法 (COMPOSITIONS AND METHODS FOR TREATING HAIR LOSS USING NON-NATURALLY OCCURRING PROSTAGLANDINS) | ⤷ Try a Trial |
| Luxembourg | 90957 | ⤷ Try a Trial | |
| Japan | 3681068 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for LATISSE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0660716 | 90957 | Luxembourg | ⤷ Try a Trial | |
| 0660716 | CA 2002 00020 | Denmark | ⤷ Try a Trial | |
| 0660716 | SPC023/2002 | Ireland | ⤷ Try a Trial | SPC023/2002: 20040929, EXPIRES: 20170307 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


