Share This Page
Drug Price Trends for LATISSE
✉ Email this page to a colleague
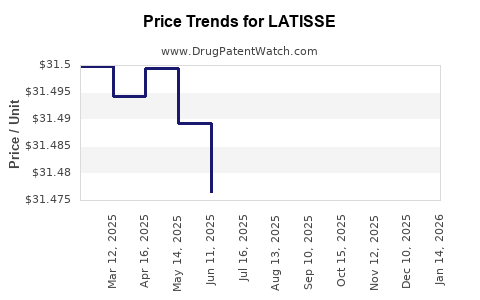
Average Pharmacy Cost for LATISSE
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| LATISSE 0.03% EYELASH SOLUTION | 00023-3616-70 | 41.01283 | ML | 2025-12-17 |
| LATISSE 0.03% EYELASH SOLUTION | 00023-3616-05 | 31.48043 | ML | 2025-12-17 |
| LATISSE 0.03% EYELASH SOLUTION | 00023-3616-70 | 41.05467 | ML | 2025-11-19 |
| LATISSE 0.03% EYELASH SOLUTION | 00023-3616-05 | 31.44643 | ML | 2025-11-19 |
| LATISSE 0.03% EYELASH SOLUTION | 00023-3616-70 | 41.11807 | ML | 2025-10-22 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Market Analysis and Price Projections for LATISSE
Introduction
LATISSE (bimatoprost ophthalmic solution) is a prescription medication developed by Allergan (recently acquired by AbbVie) for eyelash hypotrichosis, marketed primarily for enhancing eyelash length, thickness, and fullness. Since its FDA approval in December 2008, LATISSE has established itself as a dominant player in the cosmetic ophthalmic market segment, with significant implications for healthcare providers, consumers, and investors. This article provides a comprehensive market analysis and price projection for LATISSE, considering current industry trends, regulatory factors, competitive landscape, and consumer demand.
Market Overview
Historical Market Performance
LATISSE pioneered the growing eyelash enhancement segment, which initially was a niche market within cosmetic ophthalmology. Its early success was driven by patents that granted exclusivity until 2023, limiting competition and enabling premium pricing. Globally, the ophthalmic solutions market was valued at approximately USD 9.9 billion in 2022, with cosmetic ophthalmology constituting around 12%, indicating a sizable revenue stream for LATISSE [1].
Current Market Size and Growth Dynamics
The global eyelash enhancement market is projected to grow at a CAGR of approximately 7% between 2023 and 2030, driven by rising consumer interest in aesthetic enhancement, increasing awareness, and expanding application beyond licensed use—such as in lash volume treatments. North America remains the largest market, accounting for nearly 50% of sales, followed by Europe and Asia-Pacific [2].
In 2022, LATISSE's sales were estimated at USD 340 million globally, with estimates indicating a moderate decline owing to patent expiration (2023), competitive entries, and market saturation.
Competitive Landscape
Historically, LATISSE enjoyed a monopoly based on patent protections and regulatory exclusivities. However, patent cliffs and the emergence of OTC and alternative treatments threaten its market share. Notable competitors include:
- Latisse's Patent Expiration: The key composition patent expired in 2023, exposing the product to generic competition [3].
- Generic Bimatoprost: Several generic formulations have entered the market, priced approximately 35-50% lower than the brand version.
- Alternative Products: Over-the-counter serums and natural remedies are increasingly popular, although their efficacy is not clinically established.
Driving Factors Affecting Market and Pricing
Regulatory Factors
Patent expirations have led to increased generic competition, prompting pricing declines. While patent protection historically allowed LATISSE to command premium prices (~USD 120 per 3 mL bottle), generic options are now retailing at USD 60-75.
Consumer Trends
Increased awareness of eyelash aesthetics, influenced by social media and celebrity endorsements, sustains demand. However, safety concerns related to off-label use and side effects (e.g., redness, irritation) influence consumer choices.
Pricing Sensitivity and Reimbursement Dynamics
Pricing strategies are influenced by insurance coverage (limited since LATISSE is prescription-only), patient out-of-pocket costs, and the availability of cheaper generics. Clinics and practitioners may offer discounts or bundled services to retain market share.
Market Projections and Price Forecasts
Short-Term (2023-2025)
With patent expiry and the proliferation of generics, LATISSE's average sales price is projected to decline by approximately 15-20% over the next two years. From an average retail price of USD 120, expect prices to stabilize around USD 90-100 per 3 mL bottle, driven by branded product differentiation and marketing.
Medium to Long-Term (2025-2030)
Market consolidation, potential entry of biosimilars, and evolving consumer preferences forecast continued downward pressure on prices. However, the brand's strong recognition and physician recommendations could sustain premium pricing for certain segments, averaging around USD 80 per 3 mL in mature markets.
Impact of Alternative Treatments
While OTC serums currently have limited efficacy, innovations or increased consumer skepticism could slow growth. Conversely, new formulations with fewer side effects or natural ingredients might gain traction, pressuring LATISSE pricing further.
Revenue Outlook
Assuming a declining but stabilized market share, industry analysts predict LATISSE's global revenue to decline to USD 200-250 million by 2030, assuming sustained brand presence and limited market penetration of generics in premium segments.
Strategic Implications
- Pricing Strategy: Allergan/AbbVie could consider value-based pricing, emphasizing clinical efficacy and safety to differentiate from cheaper generics.
- Product Differentiation: Investment in R&D for new formulations or adjunct products may sustain premium pricing.
- Market Expansion: Tapping into emerging markets, particularly in Asia-Pacific, where aesthetic procedures are rapidly increasing, could offset declines.
Key Drivers and Risks
| Drivers | Risks |
|---|---|
| Rising consumer demand for cosmetic eyelash enhancement | Patent expiration and generic entry |
| Increasing awareness via social media | Regulatory shifts or safety concerns |
| Expansion into new markets | Competition from OTC and natural products |
Key Takeaways
- The LATISSE market is at a critical juncture following patent expiration, with significant price and volume adjustments anticipated.
- The rise of generics and OTC products will exert downward pressure on prices, especially in price-sensitive segments.
- Premium pricing will persist within established brands, contingent upon continued clinical efficacy and safety assurance.
- Strategic focus on product innovation and market expansion remains vital for maintaining profitability.
- Industry players should monitor regulatory developments and consumer trends to adapt pricing and marketing strategies effectively.
FAQs
1. What will be LATISSE’s price trend over the next five years?
Prices are expected to decline by approximately 10-20% by 2028 due to increased generic competition, stabilizing around USD 80-100 per 3 mL bottle in developed markets.
2. How does patent expiration affect LATISSE’s market share and pricing?
Patent expiry allows generic competitors, which typically price 35-50% lower, to enter the market, leading to decreased market share for the brand and downward pricing pressure.
3. Are there safe and effective alternatives to LATISSE?
Over-the-counter eyelash serums with natural ingredients are available but lack robust clinical evidence. Their safety and efficacy remain unverified, making LATISSE the preferred prescription option for consumers seeking proven results.
4. What strategies can Allergan/AbbVie adopt to sustain LATISSE’s profitability?
Investing in product innovation, emphasizing clinical efficacy, expanding into emerging markets, and developing adjunct therapies can help sustain revenue amid price erosion.
5. How might regulatory changes influence LATISSE’s future?
Risks include increased regulation of the active ingredient’s safety profile, which could impact approval of newer formulations or generics, influencing pricing and market access strategies.
References
- Market Research Future. "Global Ophthalmic Drugs Market." 2022.
- Grand View Research. "Eyelash Enhancing Products Market Size & Trends." 2023.
- U.S. Patent and Trademark Office. "Patent Expiration Dates for Bimatoprost Formulations." 2023.
- Allergan official disclosures and analyst reports. 2023.
In conclusion, the LATISSE market is undergoing transformation driven by patent expirations, evolving consumer preferences, and competitive dynamics. While prices are poised to decrease, brand recognition and incremental innovations can preserve profitability. Stakeholders must adopt adaptive strategies anchored in market intelligence and consumer insights for sustained success.
More… ↓
