KYNMOBI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Kynmobi, and when can generic versions of Kynmobi launch?
Kynmobi is a drug marketed by Sumitomo Pharma Am and is included in one NDA. There are fourteen patents protecting this drug.
This drug has two hundred and fifty-four patent family members in twenty-six countries.
The generic ingredient in KYNMOBI is apomorphine hydrochloride. There are six drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the apomorphine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Kynmobi
A generic version of KYNMOBI was approved as apomorphine hydrochloride by SAGE CHEMS on February 23rd, 2022.
Summary for KYNMOBI
| International Patents: | 254 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 1 |
| Clinical Trials: | 1 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for KYNMOBI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KYNMOBI |
| What excipients (inactive ingredients) are in KYNMOBI? | KYNMOBI excipients list |
| DailyMed Link: | KYNMOBI at DailyMed |

Recent Clinical Trials for KYNMOBI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| William Ondo, MD | Phase 4 |
| Sunovion | Phase 4 |
Anatomical Therapeutic Chemical (ATC) Classes for KYNMOBI
US Patents and Regulatory Information for KYNMOBI
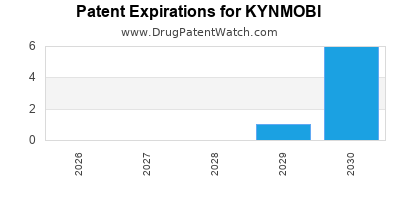
KYNMOBI is protected by fourteen US patents.
Patents protecting KYNMOBI
Sublingual apomorphine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Methods of treating parkinson's disease by administration of apomorphine to an oral mucosa
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Sublingual and buccal film compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods of treating Parkinson's disease by administration of apomorphine to an oral mucosa
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Sublingual films
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Sublingual films
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Uniform films for rapid dissolve dosage form incorporating taste-masking compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Uniform films for rapid-dissolve dosage form incorporating anti-tacking compositions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Sublingual films
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Sublingual apomorphine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Sublingual films
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Sublingual apomorphine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Sublingual apomorphine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
Sublingual apomorphine
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF 'OFF' EPISODES IN PATIENTS WITH PARKINSON'S DISEASE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-003 | May 21, 2020 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-003 | May 21, 2020 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-004 | May 21, 2020 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-005 | May 21, 2020 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-004 | May 21, 2020 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-004 | May 21, 2020 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-004 | May 21, 2020 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for KYNMOBI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-002 | May 21, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-001 | May 21, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-001 | May 21, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-004 | May 21, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-001 | May 21, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-004 | May 21, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| Sumitomo Pharma Am | KYNMOBI | apomorphine hydrochloride | FILM;SUBLINGUAL | 210875-003 | May 21, 2020 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for KYNMOBI
When does loss-of-exclusivity occur for KYNMOBI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 11343429
Estimated Expiration: ⤷ Sign Up
Patent: 17200331
Estimated Expiration: ⤷ Sign Up
Patent: 19200138
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2013015204
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 21756
Estimated Expiration: ⤷ Sign Up
Patent: 15370
Estimated Expiration: ⤷ Sign Up
Patent: 15378
Estimated Expiration: ⤷ Sign Up
Patent: 16942
Estimated Expiration: ⤷ Sign Up
China
Patent: 3476372
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 22988
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 51357
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 1156
Estimated Expiration: ⤷ Sign Up
Patent: 1390855
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 51357
Estimated Expiration: ⤷ Sign Up
Patent: 35988
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 93969
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 49349
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 5936
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 86195
Estimated Expiration: ⤷ Sign Up
Patent: 13545824
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 13006911
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 2686
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 51357
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 51357
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1304740
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1890317
Estimated Expiration: ⤷ Sign Up
Patent: 1946774
Estimated Expiration: ⤷ Sign Up
Patent: 2025238
Estimated Expiration: ⤷ Sign Up
Patent: 2161392
Estimated Expiration: ⤷ Sign Up
Patent: 140043051
Estimated Expiration: ⤷ Sign Up
Patent: 180094143
Estimated Expiration: ⤷ Sign Up
Patent: 190015597
Estimated Expiration: ⤷ Sign Up
Patent: 190109598
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 91715
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KYNMOBI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| China | 1668961 | Tunable optical instruments | ⤷ Sign Up |
| South Africa | 201507685 | SUBLINGUAL AND BUCCAL FILM COMPOSITIONS | ⤷ Sign Up |
| Canada | 3127926 | METHODES DE TRAITEMENT DE LA MALADIE DE PARKINSON PAR L'ADMINISTRATION D'APOMORPHINE A UNE MUQUEUSE ORALE (METHODS OF TREATING PARKINSON'S DISEASE BY ADMINISTRATION OF APOMORPHINE TO AN ORAL MUCOSA) | ⤷ Sign Up |
| Mexico | 2013006911 | PELICULAS SUB-LINGUALES. (SUBLINGUAL FILMS.) | ⤷ Sign Up |
| South Korea | 102025238 | ⤷ Sign Up | |
| Spain | 2791715 | ⤷ Sign Up | |
| Canada | 2468557 | EMBALLAGE DESTINE DES COMPOSANTS ELECTRO-OPTIQUES (PACKAGE FOR ELECTRO-OPTICAL COMPONENTS) | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


