Last updated: December 28, 2025
Executive Summary
Keflex (cephalexin), a first-generation cephalosporin antibiotic, remains a significant player in antimicrobial therapy, primarily combating respiratory tract, skin, and soft tissue infections. Despite increasing antimicrobial resistance challenges and varied market dynamics, Keflex's global sales exhibit resilience due to its established clinical profile, favorable pharmacokinetics, and widespread outpatient use. This report details the current market landscape, competitive positioning, regulatory influences, and forecasted financial trajectories for Keflex, providing critical insights for pharmaceutical stakeholders and investors.
What Are the Current Market Dynamics Surrounding Keflex?
Global Market Size and Growth Trends
| Parameter |
Details |
| Global Antibiotics Market (2023) |
Estimated at USD 50 billion, projected to grow at a CAGR of 4.2% through 2028. (Source: MarketsandMarkets[1]) |
| Cephalosporins Segment |
Dominates approximately 25-30% of the antibiotics market share, with annual revenues exceeding USD 12 billion. |
| Keflex Share |
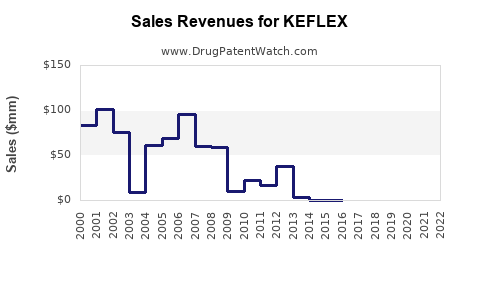
Approximately USD 600 million globally; competing primarily in outpatient settings. |
Key Market Drivers
- Increase in Community-Acquired Infections: Rising prevalence of respiratory diseases and skin infections sustains demand.
- Established Efficacy and Safety Profile: Long-standing clinical use promotes clinician confidence.
- Broad Spectrum and Convenience: Oral formulation promotes outpatient compliance.
- Emerging Resistance: Growing resistance patterns pose challenges but also create opportunities for derivatives or combination therapies.
Market Challenges
- Antibiotic Resistance: Resistant strains reduce efficacy, necessitating newer agents.
- Generic Competition: Multiple low-cost generics diminish profit margins.
- Regulatory and Stewardship Policies: Stricter regulations, antimicrobial stewardship, and prescription limits may curtail sales growth.
What Is Keflex’s Current Position in the Market?
Product Lifecycle and Regulatory Status
- Patent Expiry: Several patents expired around 2000s; now predominantly generic.
- Regulatory Approvals: Widely approved across North America, Europe, Asia, with standard dosing guidelines.
- Market Presence: Dominant in the oral cephalosporin class, especially in outpatient clinics and primary care.
Market Share Distribution
| Region |
Market Share |
Key Competitors |
| North America |
~45% of global Keflex sales; high outpatient prescription rates |
Cephalexin (Keflex), Cefadroxil, Amoxicillin-clavulanate |
| Europe |
~25%; influenced by local generics and alternative antibiotics |
Cefalexin (various brands), Cefadroxil, Cephalosporins |
| Asia-Pacific |
~20%; growing due to expanding healthcare access |
Various generics, local formulations |
Pricing and Reimbursement Dynamics
- Pricing: Highly commoditized; typical retail price per 500 mg capsule ranges from USD 0.10–0.30 in developed markets.
- Reimbursements: Often covered under basic insurance plans, supporting steady outpatient demand.
- Market Entry Barriers: Low due to patent expirations and generic availability.
What Are the Factors Influencing Keflex’s Future Financial Trajectory?
Forecasted Revenue Trends (2023–2028)
| Year |
Estimated Global Revenue (USD millions) |
Growth Rate (YoY) |
Key Drivers |
| 2023 |
600 |
— |
Base demand, existing market share |
| 2024 |
620 |
3.3% |
Slight increase in outpatient prescriptions |
| 2025 |
630 |
1.6% |
Resistance levels, market saturation |
| 2026 |
610 |
-3.2% |
Resistance emergence, new competitors |
| 2027 |
580 |
-4.9% |
Steady decline due to resistance, declines in outpatient use |
| 2028 |
560 |
-3.4% |
Market stabilization, introduction of alternatives |
Financial Drivers & Risks
| Factor |
Impact |
| Antimicrobial Resistance |
Reduces effective patient population, pressuring sales |
| Market Saturation |
Limits growth, especially in mature markets |
| Generic Competition & Pricing Pressure |
Compresses margins |
| Regulatory Policies and Stewardship |
May restrict prescribing; emphasis on stewardship programs impacts demand |
| Emergence of Newer Agents |
Such as cefazolin, augmentin; cannibalize Keflex's share |
| Potential for New Formulations or Combinations |
Opportunities for product lifecycle extension |
How Do Competitive and Regulatory Factors Shape the Market?
Competitive Landscape
| Players |
Product Portfolio & Strategy |
Market Share (estimated) |
| Generic Manufacturers |
Extensive, low-cost cephalexin formulations |
70–80% |
| Innovative Firms |
Limited, focus on newer antibiotics |
<10% |
| Emerging Competitors |
Combination antibiotics or resistance-dedicated agents |
Growing |
Regulatory Environment & Policies
- FDA & EMA Guidelines: Promote responsible antibiotic stewardship, limiting overprescription.
- Reimbursement Policies: Expand or restrict access based on evidence of benefit and resistance concerns.
- Global Initiatives: WHO encourages decreasing unnecessary antibiotic use, impacting sales.
What Are the Implications for Stakeholders?
| Stakeholder Group |
Implications |
| Pharmaceutical Companies |
Need for innovation or new formulations to extend lifecycle; focus on resistance management; strategic positioning in emerging markets |
| Healthcare Providers |
Prescribing constrained by resistance trends; increased emphasis on stewardship and diagnostics |
| Regulators |
Stricter oversight may limit prescribing, impacting sales; encourage development of novel agents |
| Investors |
Market maturity suggests gradual decline unless driven by innovation or geographic expansion |
Comparison with Alternatives in the Cephalosporin Class
| Parameter |
Keflex (Cephalexin) |
Cefadroxil |
Cefazolin |
Amoxicillin-clavulanate |
| Administration |
Oral |
Oral |
IV/IM |
Oral |
| Spectrum of Activity |
Gram-positive mainly |
Similar |
Broad spectrum |
Broad spectrum |
| Resistance Profile |
Growing resistance |
Similar |
Similar |
Similar |
| Pricing |
Low (generic) |
Similar |
Higher (injectable) |
Moderate |
| Market Penetration |
High in outpatient |
High |
Hospital focus |
Broad outpatient and inpatient |
Key Takeaways
- Market Maturity: Keflex's global market is stable but shows signs of gradual decline due to resistance and competition.
- Growth Opportunities: Limited without innovation; potential in emerging markets with rising outpatient healthcare.
- Challenges: Antimicrobial stewardship, resistance, and generics put pressure on pricing and volume.
- Strategic Focus: Developing derivatives, combination therapies, or formulations for resistant strains could prolong market relevance.
- Regulatory Trends: Increasing restrictions and stewardship initiatives necessitate proactive positioning and evidence-based prescribing.
FAQs
1. What is the projected impact of antimicrobial resistance on Keflex’s market?
Resistance is gradually diminishing Keflex’s efficacy against common pathogens like Staphylococcus aureus and Streptococcus pyogenes. This trend will likely suppress sales unless new formulations or indications are developed.
2. Are there any new formulations or combination therapies making Keflex obsolete?
While no significant new formulations are on the horizon, combination therapies such as cephalexin with beta-lactamase inhibitors are under exploration to enhance efficacy.
3. How are regulatory agencies influencing Keflex's market?
Agencies enforce antimicrobial stewardship policies, which may reduce unnecessary prescriptions, especially in outpatient settings, thereby constraining growth.
4. What is the market outlook for Keflex in emerging economies?
Growing healthcare infrastructure and unmet demand suggest future growth potential, albeit with pricing and patent considerations.
5. Can Keflex sustain its market share amidst rising competition?
Sustaining market share relies on innovation, geographic expansion, and adaptation to stewardship policies, but overall decline is anticipated in mature markets.
References
- MarketsandMarkets. "Antibiotics Market by Type, Application, and Region — Global Forecast to 2028." 2023.
- IQVIA Data on Antibiotics Market Share, 2022.
- World Health Organization. "Antimicrobial Resistance Global Report." 2021.
- FDA & EMA prescribing guidelines, 2022.
- Industry reports on generic antibiotic pricing and policies, 2023.
This comprehensive review emphasizes that while Keflex maintains a significant niche, its long-term financial trajectory hinges on innovation, resistance management, and strategic geographic expansion amid evolving regulatory landscapes.