KALYDECO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Kalydeco, and what generic alternatives are available?
Kalydeco is a drug marketed by Vertex Pharms Inc and Vertex Pharms and is included in two NDAs. There are thirteen patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and fifty-three patent family members in thirty-two countries.
The generic ingredient in KALYDECO is ivacaftor. There are three drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the ivacaftor profile page.
DrugPatentWatch® Generic Entry Outlook for Kalydeco
Kalydeco was eligible for patent challenges on January 31, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 13, 2029. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (ivacaftor), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for KALYDECO
| International Patents: | 253 |
| US Patents: | 13 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 104 |
| Clinical Trials: | 26 |
| Patent Applications: | 806 |
| Drug Prices: | Drug price information for KALYDECO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for KALYDECO |
| What excipients (inactive ingredients) are in KALYDECO? | KALYDECO excipients list |
| DailyMed Link: | KALYDECO at DailyMed |
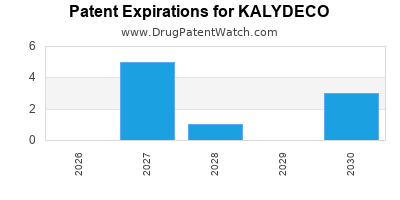

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for KALYDECO
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for KALYDECO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | Phase 2 |
| University of Kansas Medical Center | Early Phase 1 |
| University of North Carolina | Early Phase 1 |
Pharmacology for KALYDECO
Anatomical Therapeutic Chemical (ATC) Classes for KALYDECO
US Patents and Regulatory Information for KALYDECO
KALYDECO is protected by thirty-two US patents and seven FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of KALYDECO is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting KALYDECO
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 1 MONTH TO
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 4 MONTHS TO
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 6 MONTHS TO
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 1 MONTH TO
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 4 MONTHS TO
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 1 MONTH TO
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 4 MONTHS TO
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 6 YEARS AND OLDER WHO HAS ONE MUTATION IN THE CFTR GENE THAT IS RESPONSIVE TO IVACAFTOR BASED ON CLINICAL AND/OR IN VITRO ASSAY DATA USING THE COMPOSITION RECITED IN CLAIM 1 OF US 11564916
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 1 MONTH TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS USING IVACAFTOR IN A PATIENT AGE 1 MONTH TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING A PATIENT HAVING CYSTIC FIBROSIS, SUCH AS A PATIENT HAVING A G551D MUTATION IN CFTR, USING N-(5-HYDROXY-2,4-DI-TERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS USING IVACAFTOR IN A PATIENT AGE 4 MONTHS TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS USING IVACAFTOR IN A PATIENT AGE 6 MONTHS TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS USING IVACAFTOR IN A PATIENT AGE 1 MONTH TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING A PATIENT HAVING CYSTIC FIBROSIS, THE PATIENT HAVING A R117H MUTATION IN CFTR, USING N-(5-HYDROXY-2,4-DI-TERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS USING IVACAFTOR IN A PATIENT AGE 4 MONTHS TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CYSTIC FIBROSIS USING IVACAFTOR IN A PATIENT AGE 6 MONTHS TO
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A MODERATE TO MILD CLINICAL PHENOTYPE OF CF USING IVACAFTOR IN A PATIENT AGE 1 MONTH TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: USE OF IVACAFTOR FOR TREATING CYSTIC FIBROSIS IN A PATIENT WITH A MILD TO MODERATE CF PHENOTYPE WITH AT LEAST ONE MUTATION IN THE CFTR GENE THAT IS RESPONSIVE TO IVACAFTOR BASED ON CLINICAL AND/OR IN VITRO ASSAY DATA
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A MODERATE TO MILD CLINICAL PHENOTYPE OF CF USING IVACAFTOR IN A PATIENT AGE 4 MONTHS TO
Modulators of ATP-binding cassette transporters
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF A MODERATE MILD CLINICAL PHENOTYPE OF CF USING IVACAFTOR IN A PATIENT AGE 6 MONTHS TO
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Pharmaceutical composition and administrations thereof
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 1 MONTH TO
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: METHOD OF TREATING CYSTIC FIBROSIS
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 4 MONTHS TO
Solid forms of N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxoquinoline- -3-carboxamide
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF CF IN A PATIENT AGE 6 MONTHS TO < 6 YEARS WHO HAS ONE CFTR MUTATION RESPONSIVE TO IVACAFTOR BASED ON CLINICAL AND/OR IN VITRO ASSAY DATA USING A SOLID COMPOSITION COMPRISING AMORPHOUS (LESS THAN ABOUT 30% CRYSTALLINE) IVACAFTOR
FDA Regulatory Exclusivity protecting KALYDECO
THE TREATMENT OF CYSTIC FIBROSIS (CF) IN PATIENTS 1 MONTH TO LESS THAN 4 MONTHS OF AGE WHO HAVE AT LEAST ONE MUTATION IN THE CFTR GENE THAT IS RESPONSIVE TO IVACAFTOR POTENTIATION BASED ON CLINICAL AND/OR IN VITRO ASSAY DATA
Exclusivity Expiration: ⤷ Sign Up
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF CYSTIC FIBROSIS (CF) IN PATIENTS AGE 6 MONTHS TO LESS THAN 12 MONTHS WHO HAVE ONE MUTATION IN THE CFTR GENE THAT IS RESPONSIVE TO IVACAFTOR POTENTIATION BASED ON CLINICAL AND/OR IN VITRO ASSAY DATA
Exclusivity Expiration: ⤷ Sign Up
TX OF CF IN PTS 2 YRS AND OLDER WHO HAVE ONE OF THE FOLLOWING MUTATIONS IN THE CFTR GENE: E56K, P67L, R74W, D110E, D110H, R117C, E193K, L206W, R347H, R352Q, A455E, D579G, S945L, S977F, F1052V, K1060T, A1067T, G1069R, R1070Q, R1070W, F1074L, D1152H, D1270N
Exclusivity Expiration: ⤷ Sign Up
THE TREATMENT OF CYSTIC FIBROSIS (CF) IN PATIENTS AGE 12 MONTHS AND OLDER WHO HAVE ONE MUTATION IN THE CFTR GENE THAT IS RESPONSIVE TO IVACAFTOR POTENTIATION BASED ON CLINICAL AND/OR IN VITRO ASSAY DATA
Exclusivity Expiration: ⤷ Sign Up
TREATMENT OF CYSTIC FIBROSIS (CF) IN PATIENTS AGE 2 YEARS AND OLDER WHO HAVE ONE OF THE FOLLOWING MUTATIONS IN THE CFTR GENE: 711+3A-G, E831X, 2789+5G-A, 3272-26A-G, AND 3849+10KBC-T
Exclusivity Expiration: ⤷ Sign Up
FOR THE TREATMENT OF CYSTIC FIBROSIS (CF) IN PATIENTS AGE 4 MONTHS AND OLDER WHO HAVE ONE OF THE ADDITIONAL MUTATIONS IN THE CYSTIC FIBROSIS TRANSMEMBRANE CONDUCTANCE REGULATOR (CFTR) GENE THAT HAVE BEEN IDENTIFIED AS RESPONSIVE TO IVACAFTOR POTENTIATION BASED ON IN VITRO DATA AND IDENTIFIED IN THE APPROVAL ON DECEMBER 21, 2020
Exclusivity Expiration: ⤷ Sign Up
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-001 | Mar 17, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-002 | Mar 17, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-003 | Apr 29, 2019 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-002 | Mar 17, 2015 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Vertex Pharms | KALYDECO | ivacaftor | TABLET;ORAL | 203188-001 | Jan 31, 2012 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-003 | Apr 29, 2019 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Vertex Pharms Inc | KALYDECO | ivacaftor | GRANULE;ORAL | 207925-001 | Mar 17, 2015 | RX | Yes | No | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for KALYDECO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Vertex Pharmaceuticals (Ireland) Limited | Kalydeco | ivacaftor | EMEA/H/C/002494 Kalydeco tablets are indicated:As monotherapy for the treatment of adults, adolescents, and children aged 6 years and older and weighing 25 kg or more with cystic fibrosis (CF) who have an R117H CFTR mutation or one of the following gating (class III) mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R (see sections 4.4 and 5.1).In a combination regimen with tezacaftor/ivacaftor tablets for the treatment of adults, adolescents, and children aged 6 years and older with cystic fibrosis (CF) who are homozygous for the F508del mutation or who are heterozygous for the F508del mutation and have one of the following mutations in the CFTR gene: P67L, R117C, L206W, R352Q, A455E, D579G, 711+3A→G, S945L, S977F, R1070W, D1152H, 2789+5G→A, 3272 26A→G, and 3849+10kbC→T.In a combination regimen with ivacaftor/tezacaftor/elexacaftor tablets for the treatment of adults, adolescents, and children aged 6 years and older with cystic fibrosis (CF) who have at least one F508del mutation in the CFTR gene (see section 5.1).Kalydeco granules are indicated for the treatment of infants aged at least 4 months, toddlers and children weighing 5 kg to less than 25 kg with cystic fibrosis (CF) who have an R117H CFTR mutation or one of the following gating (class III) mutations in the CFTR gene: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R (see sections 4.4 and 5.1). |
Authorised | no | no | no | 2012-07-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for KALYDECO
When does loss-of-exclusivity occur for KALYDECO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 09282419
Estimated Expiration: ⤷ Sign Up
Patent: 10282986
Estimated Expiration: ⤷ Sign Up
Patent: 13226076
Estimated Expiration: ⤷ Sign Up
Patent: 16216569
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2014021090
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 65519
Estimated Expiration: ⤷ Sign Up
China
Patent: 4470518
Estimated Expiration: ⤷ Sign Up
Patent: 9966264
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 19670
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 03840
Estimated Expiration: ⤷ Sign Up
Patent: 05690
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 4307
Estimated Expiration: ⤷ Sign Up
Patent: 5430
Estimated Expiration: ⤷ Sign Up
Patent: 1180
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 34041
Estimated Expiration: ⤷ Sign Up
Patent: 15511583
Estimated Expiration: ⤷ Sign Up
Patent: 17190356
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 14010253
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 9199
Estimated Expiration: ⤷ Sign Up
Russian Federation
Patent: 92779
Estimated Expiration: ⤷ Sign Up
Patent: 14139006
Estimated Expiration: ⤷ Sign Up
Patent: 19116577
Estimated Expiration: ⤷ Sign Up
South Africa
Patent: 1406233
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering KALYDECO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Ukraine | 102261 | ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ НА ОСНОВІ ТВЕРДОЇ ДИСПЕРСІЇ N-[2,4-БІС(1,1-ДИМЕТИЛЕТИЛ)-5-ГІДРОКСИФЕНІЛ]-1,4-ДИГІДРО-4-ОКСОХІНОЛІН-3-КАРБОКСАМІДУ[ФАРМАЦЕВТИЧЕСКИЕ КОМПОЗИЦИЯ НА ОСНОВЕ ТВЕРДОЙ ДИСПЕРСИИ N-[2,4-БИС(1,1-ДИМЕТИЛЭТИЛ)-5-ГИДРОКСИФЕНИЛ]-1,4-ДИГИДРО-4-ОКСОХИНОЛИН-3-КАРБОКСАМИДА (PHARMACEUTICAL COMPOSITIONS COMPRISING A SOLID DISPERSION OF N-[2,4-BIS(1,1-DIMETHYLETHYL)-5-HYDROXYPHENYL]-1,4-DIHYDRO-4-OXOQUINOLINE-3-CARBOXAMIDE) | ⤷ Sign Up |
| Denmark | 2502911 | ⤷ Sign Up | |
| Lithuania | 1993360 | ⤷ Sign Up | |
| Canada | 2635581 | FORMES SOLIDES DE N-[2,4-BIS(1,1-DIMETHYLETHYL)-5-HYDROXYPHENYL]-1,4-DIHYDRO-4-OXOQUINOLEINE-3-CARBOXAMIDE (SOLID FORMS OF N-[2,4-BIS(1,1-DIMETHYLETHYL)-5-HYDROXYPHENYL]-1,4-DIHYDRO-4-OXOQUINOLINE-3-CARBOXAMIDE) | ⤷ Sign Up |
| Russian Federation | 2009141996 | МОДУЛЯТОРЫ ТРАНСПОРТЕРОВ АТФ-СВЯЗЫВАЮЩЕЙ КАССЕТЫ | ⤷ Sign Up |
| New Zealand | 598393 | Modulators of atp-binding cassette transporters | ⤷ Sign Up |
| Australia | 2010327993 | 4-oxo-1H-quinoline-3-carboxamides as modulators of ATP-Binding cassette transporters | ⤷ Sign Up |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for KALYDECO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3170818 | C202030042 | Spain | ⤷ Sign Up | PRODUCT NAME: UNA COMBINACION DE A) LUMACAFTOR Y B) IVACAFTOR; NATIONAL AUTHORISATION NUMBER: EU/1/15/1059; DATE OF AUTHORISATION: 20151119; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/15/1059; DATE OF FIRST AUTHORISATION IN EEA: 20151119 |
| 1773816 | 92761 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: N-(5-HYDROXY-2,4-DIERT-BUTYL-PHENYL)-4OXO-1H-QUINOLINE-3-CARBOXAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; FIRST REGISTRATION: 20120725 |
| 1773816 | PA2015028 | Lithuania | ⤷ Sign Up | PRODUCT NAME: IVACAFTORUM; REGISTRATION NO/DATE: EU/1/12/782/001 - EU/1/12/782/002 20120723 |
| 2826776 | 132021000000062 | Italy | ⤷ Sign Up | PRODUCT NAME: UNA COMBINAZIONE DI (A) TEZACAFTOR O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE E (B) IVACAFTOR O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE(SYMKEVI); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/18/1306, 20181106 |
| 2826776 | 2021C/517 | Belgium | ⤷ Sign Up | PRODUCT NAME: SYMKEVI - TEZACAFTOR/IVACAFTOR; EEN COMBINATIE VAN (A) (R)-1-(2,2-DIFLUOROBENZO(D)(1,3)DIOXOL-5-YL)-N-(1-(2,3-DIHYDROXYPROPYL)-6-FLUORO-2-(1-HYDROXY-2-METHYLPROPAN-2-YL)-1H-INDOL-5-YL)CYCLOPROPANECARBOXAMIDE OF EEN VANUIT FARMACEUTISCH OOGPUNT GESCHIKT ZOUT DAARVAN EN (B) N-(5-HYDROXY-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3-CARBOXAMIDE OF EEN VANUIT FARMACEUTISCH OOGPUNT GESCHIKT ZOUT DAARVAN; AUTHORISATION NUMBER AND DATE: EU/1/18/1306 20181106 |
| 1773816 | C300748 | Netherlands | ⤷ Sign Up | PRODUCT NAME: N-(5-HYDROXY-2,4-DI-TERT-BUTYL-; REGISTRATION NO/DATE: EU/1/12/782/001-002 20120725 |
| 1773816 | 2015/036 | Ireland | ⤷ Sign Up | PRODUCT NAME: N-(5-HYDROXYL-2,4-DITERT-BUTYL-PHENYL)-4-OXO-1H-QUINOLINE-3- CARBOXAMIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTRATION NO/DATE: EU/1/12/782/001-002 20120723 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


