JARDIANCE Drug Patent Profile
✉ Email this page to a colleague
When do Jardiance patents expire, and what generic alternatives are available?
Jardiance is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are fourteen patents protecting this drug and one Paragraph IV challenge.
This drug has three hundred and seventy-eight patent family members in forty-six countries.
The generic ingredient in JARDIANCE is empagliflozin. There are twenty-two drug master file entries for this compound. Four suppliers are listed for this compound. Additional details are available on the empagliflozin profile page.
DrugPatentWatch® Generic Entry Outlook for Jardiance
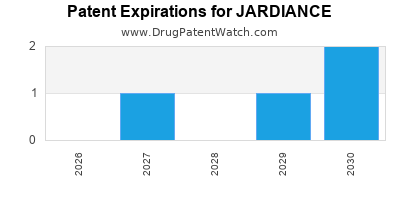
Jardiance was eligible for patent challenges on August 1, 2018.
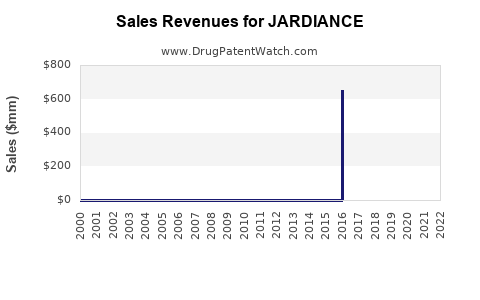
Annual sales in 2022 were $13.0bn, indicating a strong incentive for generic entry.
There have been forty-six patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are seventeen tentative approvals for the generic drug (empagliflozin), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for JARDIANCE?
- What are the global sales for JARDIANCE?
- What is Average Wholesale Price for JARDIANCE?
Summary for JARDIANCE
| International Patents: | 378 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 4 |
| Raw Ingredient (Bulk) Api Vendors: | 81 |
| Clinical Trials: | 82 |
| Patent Applications: | 2,158 |
| Drug Prices: | Drug price information for JARDIANCE |
| Drug Sales Revenues: | Drug sales revenues for JARDIANCE |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for JARDIANCE |
| What excipients (inactive ingredients) are in JARDIANCE? | JARDIANCE excipients list |
| DailyMed Link: | JARDIANCE at DailyMed |
Recent Clinical Trials for JARDIANCE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Boehringer Ingelheim | PHASE1 |
| Odense University Hospital | PHASE4 |
| The Pittsburgh Foundation | PHASE4 |
Pharmacology for JARDIANCE
| Drug Class | Sodium-Glucose Cotransporter 2 Inhibitor |
| Mechanism of Action | Sodium-Glucose Transporter 2 Inhibitors |
Paragraph IV (Patent) Challenges for JARDIANCE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| JARDIANCE | Tablets | empagliflozin | 10 mg and 25 mg | 204629 | 14 | 2018-08-01 |
US Patents and Regulatory Information for JARDIANCE
JARDIANCE is protected by fourteen US patents and five FDA Regulatory Exclusivities.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | JARDIANCE | empagliflozin | TABLET;ORAL | 204629-002 | Aug 1, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Boehringer Ingelheim | JARDIANCE | empagliflozin | TABLET;ORAL | 204629-002 | Aug 1, 2014 | RX | Yes | Yes | 11,833,166*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Boehringer Ingelheim | JARDIANCE | empagliflozin | TABLET;ORAL | 204629-002 | Aug 1, 2014 | RX | Yes | Yes | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Boehringer Ingelheim | JARDIANCE | empagliflozin | TABLET;ORAL | 204629-002 | Aug 1, 2014 | RX | Yes | Yes | 12,115,179*PED | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for JARDIANCE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim International GmbH | Jardiance | empagliflozin | EMEA/H/C/002677Type 2 diabetes mellitusJardiance is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exerciseas monotherapy when metformin is considered in addition to other medicinal products for the treatment of diabetesFor study results with respect to combinations of therapies, effects on glycaemic control, and cardiovascular and renal events, and the populations studied, see sections 4.4, 4.5 and 5.1. of the annex.Heart failureJardiance is indicated in adults for the treatment of symptomatic chronic heart failure. Chronic kidney diseaseJardiance is indicated in adults for the treatment of chronic kidney disease. | Authorised | no | no | no | 2014-05-22 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for JARDIANCE
When does loss-of-exclusivity occur for JARDIANCE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 7970
Estimated Expiration: ⤷ Get Started Free
Patent: 7657
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 08288407
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 0815331
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 96558
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 08002427
Estimated Expiration: ⤷ Get Started Free
China
Patent: 1784270
Estimated Expiration: ⤷ Get Started Free
Patent: 4288166
Estimated Expiration: ⤷ Get Started Free
Patent: 4353077
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 51239
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0170022
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 18308
Estimated Expiration: ⤷ Get Started Free
Patent: 17017
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 87879
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 109977
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8608
Estimated Expiration: ⤷ Get Started Free
Patent: 1000321
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 87879
Estimated Expiration: ⤷ Get Started Free
Patent: 98152
Estimated Expiration: ⤷ Get Started Free
Patent: 06156
Estimated Expiration: ⤷ Get Started Free
Patent: 39577
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 01721
Estimated Expiration: ⤷ Get Started Free
Patent: 03351
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 30158
Estimated Expiration: ⤷ Get Started Free
Patent: 700020
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 2886
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 95914
Estimated Expiration: ⤷ Get Started Free
Patent: 10535850
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 187879
Estimated Expiration: ⤷ Get Started Free
Patent: 2017014
Estimated Expiration: ⤷ Get Started Free
Patent: 87879
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 2037
Patent: PHARMACEUTICAL COMPOSITION COMPRISING A GLUCOPYRANOSYL-SUBSTITUTED BENZENE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 10001696
Patent: COMPOSICION FARMACEUTICA QUE COMPRENDE UN DERIVADO DE BENCENO SUSTITUIDO CON GLUCOPIRANOSILO. (PHARMACEUTICAL COMPOSITION COMPRISING A GLUCOPYRANOSYL-SUBSTITUTE D BENZENE DERIVATIVE.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 573
Patent: FARMACEUTSKA KOMPOZICIJA KOJA SADRŽI GLUKOPIRANOZILOM SUPSTITUISANI DERIVAT BENZENA (PHARMACEUTICAL COMPOSITION COMPRISING A GLUCOPYRANOSYL-SUBSTITUTED BENZENE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 612
Patent: COMPOSITION PHARMACEUTIQUE CONTENANT UN DERIVE DE BENZENE SUBSTITUE PAR GLUCOPYRANOSYLE
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 0872
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3242
Patent: PHARMACEUTICAL COMPOSITION COMPRISING A GLUCOPYRANOSYL-SUBSTITUTED BENZENE DERIVATIVE
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 17020
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 090938
Patent: COMPOSICION FARMACEUTICA QUE COMPRENDE UN DERIVADO DE BENCENO SUSTITUIDO CON GLUCOPIRANOSILO
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 87879
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 87879
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 205
Patent: FARMACEUTSKA KOMPOZICIJA KOJA SADRŽI GLUKOPIRANOZILOM SUPSTITUISANI DERIVAT BENZENA (PHARMACEUTICAL COMPOSITION COMPRISING A GLUCOPYRANOSYL-SUBSTITUTED BENZENE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 87879
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 0909105
Patent: Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivative
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1491554
Estimated Expiration: ⤷ Get Started Free
Patent: 100049595
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 02748
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 72325
Estimated Expiration: ⤷ Get Started Free
Patent: 0914030
Patent: Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivative
Estimated Expiration: ⤷ Get Started Free
Patent: 1436798
Patent: Pharmaceutical composition comprising a glucopyranosyl-substituted benzene derivative
Estimated Expiration: ⤷ Get Started Free
Tunisia
Patent: 10000073
Patent: COMPOSITON PHARMACEUTIQUE CONTENANT UN DERIVE DE BENZENE SUBSTITUE PAR GLUCOPYRANOSYLE
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 0384
Patent: ФАРМАЦЕВТИЧНА КОМПОЗИЦІЯ, ЩО МІСТИТЬ ГЛЮКОПІРАНОЗИЛЗАМІЩЕНУ ПОХІДНУ БЕНЗОЛУ ТА ІНГІБІТОР DPP IV (PHARMACEUTICAL COMPOSITION COMPRISING A GLUCOPYRANOSYL-SUBSTITUTED BENZENE DERIVATIVE)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 296
Patent: COMPOSICION FARMACÉUTICA QUE COMPRENDE UN DERIVADO DE BENCENO SUSTITUIDO CON GLUCOPIRANOSILO
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering JARDIANCE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 7454531 | ⤷ Get Started Free | |
| Taiwan | 200538463 | The glucopyranosyl-substituted phenyl derivatives | ⤷ Get Started Free |
| Hong Kong | 1215398 | ⤷ Get Started Free | |
| Japan | 2017186371 | エンパグリフロジンの治療的使用 (THERAPEUTIC USES OF EMPAGLIFLOZIN) | ⤷ Get Started Free |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for JARDIANCE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2187879 | 17C1016 | France | ⤷ Get Started Free | PRODUCT NAME: COMBINAISON D'EMPAGLIFLOZINE ET DE LINAGLIPTINE OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/16/1146 20161115 |
| 1730131 | CA 2014 00054 | Denmark | ⤷ Get Started Free | PRODUCT NAME: EMPAGLIFLOZIN OG SALTE DERAF, SAERLIGT EMPAGLIFLOZIN; REG. NO/DATE: EU1/14/930/001/018 20140522 |
| 1730131 | 200 5026-2014 | Slovakia | ⤷ Get Started Free | PRODUCT NAME: EMPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/14/930 20140527 |
| 1730131 | 14C0074 | France | ⤷ Get Started Free | PRODUCT NAME: EMPAGLIFLOZINE ET SES SELS, EN PARTICULIER L'EMPAGLIFLOZINE; REGISTRATION NO/DATE: EU/1/14/930 20140527 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for JARDIANCE (Empagliflozin)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
