INTELENCE Drug Patent Profile
✉ Email this page to a colleague
When do Intelence patents expire, and what generic alternatives are available?
Intelence is a drug marketed by Janssen R And D and is included in one NDA.
The generic ingredient in INTELENCE is etravirine. There are five drug master file entries for this compound. Five suppliers are listed for this compound. Additional details are available on the etravirine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Intelence
A generic version of INTELENCE was approved as etravirine by AMNEAL on June 14th, 2021.
Summary for INTELENCE
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 120 |
| Clinical Trials: | 14 |
| Patent Applications: | 4,021 |
| Formulation / Manufacturing: | see details |
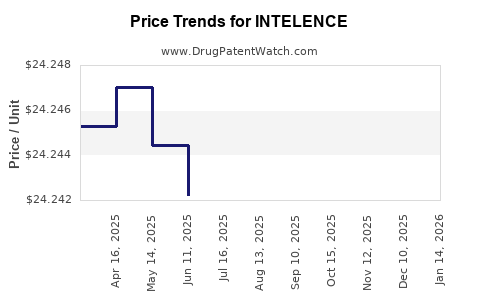
| Drug Prices: | Drug price information for INTELENCE |
| What excipients (inactive ingredients) are in INTELENCE? | INTELENCE excipients list |
| DailyMed Link: | INTELENCE at DailyMed |
Recent Clinical Trials for INTELENCE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| IRCCS Eugenio Medea | Phase 2 |
| University of Rome Tor Vergata | Phase 2 |
| Fundación FLS de Lucha Contra el Sida, las Enfermedades Infecciosas y la Promoción de la Salud y la Ciencia | Phase 1 |
Pharmacology for INTELENCE
Anatomical Therapeutic Chemical (ATC) Classes for INTELENCE
US Patents and Regulatory Information for INTELENCE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen R And D | INTELENCE | etravirine | TABLET;ORAL | 022187-003 | Mar 26, 2012 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen R And D | INTELENCE | etravirine | TABLET;ORAL | 022187-001 | Jan 18, 2008 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Janssen R And D | INTELENCE | etravirine | TABLET;ORAL | 022187-002 | Dec 22, 2010 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for INTELENCE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen R And D | INTELENCE | etravirine | TABLET;ORAL | 022187-001 | Jan 18, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen R And D | INTELENCE | etravirine | TABLET;ORAL | 022187-003 | Mar 26, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Janssen R And D | INTELENCE | etravirine | TABLET;ORAL | 022187-003 | Mar 26, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for INTELENCE
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International NV | Intelence | etravirine | EMEA/H/C/000900 Intelence, in combination with a boosted protease inhibitor and other antiretroviral medicinal products, is indicated for the treatment of human-immunodeficiency-virus-type-1 (HIV-1) infection in antiretroviral-treatment-experienced adult patients and in antiretroviral-treatment-experienced paediatric patients from six years of age.This indication is based on week-48 analyses from two phase-III trials in highly pretreated patients where Intelence was investigated in combination with an optimised background regimen (OBR) which included darunavir/ritonavir. The indication in paediatric patients is based on 48-week analyses of a single-arm, phase-II trial in antiretroviral-treatment-experienced paediatric patients. |
Authorised | no | no | no | 2008-08-28 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for INTELENCE
See the table below for patents covering INTELENCE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Norway | 333358 | ⤷ Try a Trial | |
| Bulgaria | 105418 | ⤷ Try a Trial | |
| Australia | 2011200708 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for INTELENCE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1002795 | C01002795/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: ETRAVIRIN; REGISTRATION NUMBER/DATE: SWISSMEDIC 58483 13.05.2008 |
| 1002795 | PA2008016,C1002795 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ETRAVIRINUM; REGISTRATION NO/DATE: EU/1/08/468/001 20080828 |
| 1002795 | 122009000003 | Germany | ⤷ Try a Trial | PRODUCT NAME: ETRAVIRIN UND DESSEN ADDITIONSSALZE; REGISTRATION NO/DATE: EU/1/08/468/001 20080828 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


