HARVONI Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Harvoni, and when can generic versions of Harvoni launch?
Harvoni is a drug marketed by Gilead Sciences Inc and is included in two NDAs. There are seventeen patents protecting this drug.
This drug has five hundred and sixty-four patent family members in forty-nine countries.
The generic ingredient in HARVONI is ledipasvir; sofosbuvir. There is one drug master file entry for this compound. Two suppliers are listed for this compound. Additional details are available on the ledipasvir; sofosbuvir profile page.
DrugPatentWatch® Generic Entry Outlook for Harvoni
Harvoni was eligible for patent challenges on October 10, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 14, 2032. This may change due to patent challenges or generic licensing.
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for HARVONI?
- What are the global sales for HARVONI?
- What is Average Wholesale Price for HARVONI?
Summary for HARVONI
| International Patents: | 564 |
| US Patents: | 17 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 36 |
| Drug Prices: | Drug price information for HARVONI |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for HARVONI |
| What excipients (inactive ingredients) are in HARVONI? | HARVONI excipients list |
| DailyMed Link: | HARVONI at DailyMed |
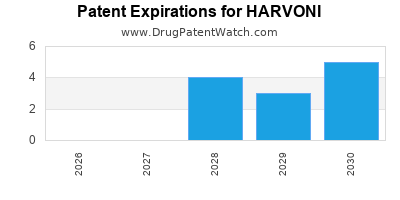

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for HARVONI
Generic Entry Dates for HARVONI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
PELLETS;ORAL |
Generic Entry Dates for HARVONI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for HARVONI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Mansoura University | Phase 3 |
| Cairo University | Phase 2/Phase 3 |
| University of Maryland, College Park | Early Phase 1 |
Pharmacology for HARVONI
US Patents and Regulatory Information for HARVONI
HARVONI is protected by seventeen US patents and four FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of HARVONI is ⤷ Get Started Free.
This potential generic entry date is based on patent 10,456,414.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
International Patents for HARVONI
When does loss-of-exclusivity occur for HARVONI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8580
Patent: METODOS Y COMPOSICIONES PARA TRATAR EL VIRUS DE LA HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Patent: 9578
Patent: COMPOSICIONES, METODOS PARA TRATAR EL VIRUS DE LA HEPATITIS C Y PROCESO DE PREPARACION
Estimated Expiration: ⤷ Get Started Free
Patent: 5131
Patent: FORMULACIÓN COMBINADA DE DOS COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 0558
Patent: COMPOSICIÓN FARMACÉUTICA QUE COMPRENDE LEDIPASVIR Y SOFOSBUVIR, FORMA DE DOSIFICACIÓN FARMACÉUTICA Y COMPRIMIDO QUE LA COMPRENDEN
Estimated Expiration: ⤷ Get Started Free
Australia
Patent: 12308295
Patent: Methods for treating HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 12332827
Patent: Methods and compositions for treating hepatitis C virus
Estimated Expiration: ⤷ Get Started Free
Patent: 12346217
Patent: Compositions and methods for treating hepatitis C virus
Estimated Expiration: ⤷ Get Started Free
Patent: 14202687
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 15202842
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 16216641
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 18203696
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014006324
Patent: método para tratar hcv
Estimated Expiration: ⤷ Get Started Free
Patent: 2014010295
Patent: métodos e composições para o tratamento de vírus de hepatite c
Estimated Expiration: ⤷ Get Started Free
Patent: 2014011938
Patent: combinação de formulação de dois compostos antivirais
Estimated Expiration: ⤷ Get Started Free
Patent: 2014012739
Patent: composições e métodos para o tratamento do vírus da hepatite c
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 40242
Patent: PROCEDES PERMETTANT DE TRAITER LE VIRUS DE L'HEPATITE C (HCV) (METHODS FOR TREATING HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 52867
Patent: COMBINAISON DE FORMULATION DE DEUX COMPOSES ANTIVIRAUX (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 53495
Patent: METHODES ET COMPOSITIONS POUR LE TRAITEMENT DU VIRUS DE L'HEPATITE C (METHODS AND COMPOSITIONS FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Patent: 56529
Patent: COMPOSITIONS ET METHODES POUR TRAITER LE VIRUS DE L'HEPATITE C (COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14000630
Patent: Una composicion que comprende 1) un compuesto derivado de nucleosido denominado como compuesto 10 y 2) un compuesto derivado de acido 5-tiofeno-2-carboxilico denominado como compuesto 5 o un compuesto derivado del ester metilico del acido carbamico denominado como compuesto 6; y su uso para el tratamiento profilactico o terapeutico de una infeccion por vhc.
Estimated Expiration: ⤷ Get Started Free
Patent: 14001397
Patent: Composicion farmaceutica que comprende a) gs-7977 y b) al menos un excipiente farmaceuticamente estable; forma de dosificacion unitaria; proceso de preparacion de la composicion; uso de la composicion para tratar una infeccion causada por el virus de la hepatitis c.
Estimated Expiration: ⤷ Get Started Free
Patent: 15002164
Patent: Formulación combinada de dos compuesos antivirales.
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4039319
Patent: Compositions and methods for treating hepatitis c virus
Estimated Expiration: ⤷ Get Started Free
Patent: 4144682
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 4244945
Patent: METHODS FOR TREATING HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 4244947
Patent: METHODS AND COMPOSITIONS FOR TREATING HEPATITIS C VIRUS
Estimated Expiration: ⤷ Get Started Free
Patent: 5748499
Patent: 两个抗病毒化合物的联用制剂 (Combination formulation of two antiviral compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 6166160
Patent: 用于治疗HCV的组合物 (Composition for treating HCV)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 30366
Patent: Método para el tratamiento de vhc
Estimated Expiration: ⤷ Get Started Free
Patent: 70603
Patent: Composiciones y métodos para tratar el virus de la hepatitis c
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 140177
Patent: MÉTODOS PARA EL TRATAMIENTO DE VHC
Estimated Expiration: ⤷ Get Started Free
Patent: 140273
Patent: COMPOSICIONES Y MÉTODOS PARA TRATAR EL VIRUS DE LA HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Patent: 150453
Patent: FORMULACIÓN COMBINADA DE DOS COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0180237
Estimated Expiration: ⤷ Get Started Free
Patent: 0200138
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 19896
Estimated Expiration: ⤷ Get Started Free
Patent: 22718
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 09613
Estimated Expiration: ⤷ Get Started Free
Patent: 50786
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 14005678
Patent: COMPOSICIONES Y MÉTODOS PARA TRATAR EL VIRUS DE LA HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Patent: 14013312
Patent: COMPOSICIONES PARA EL TRATAMIENTO DE VHC
Estimated Expiration: ⤷ Get Started Free
Patent: 15037311
Patent: FORMULACIÓN COMBINADA DE DOS COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Patent: 21087299
Patent: COMPOSICIONES Y MÉTODOS PARA TRATAR EL VIRUS DE LA HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 6667
Patent: ФАРМАЦЕВТИЧЕСКАЯ КОМПОЗИЦИЯ ДЛЯ ЛЕЧЕНИЯ ВИРУСА ГЕПАТИТА С (PHARMACEUTICAL COMPOSITION FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Patent: 7296
Patent: КОМПОЗИЦИИ И СПОСОБЫ ЛЕЧЕНИЯ ВИРУСНОГО ГЕПАТИТА C (COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Patent: 9081
Patent: КОМБИНИРОВАННЫЙ СОСТАВ ДВУХ ПРОТИВОВИРУСНЫХ СОЕДИНЕНИЙ (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 1490588
Patent: СПОСОБЫ ЛЕЧЕНИЯ ВИРУСА ГЕПАТИТА С
Estimated Expiration: ⤷ Get Started Free
Patent: 1490806
Patent: КОМБИНИРОВАННЫЙ СОСТАВ ДВУХ ПРОТИВОВИРУСНЫХ СОЕДИНЕНИЙ
Estimated Expiration: ⤷ Get Started Free
Patent: 1490903
Patent: КОМПОЗИЦИИ И СПОСОБЫ ЛЕЧЕНИЯ ВИРУСНОГО ГЕПАТИТА C
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 09613
Patent: PROCÉDÉS PERMETTANT DE TRAITER LE VIRUS DE L'HÉPATITE C (HCV) (METHODS FOR TREATING HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 76024
Patent: MÉTHODES ET COMPOSITIONS POUR LE TRAITEMENT DU VIRUS DE L'HÉPATITE C (METHODS AND COMPOSITIONS FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Patent: 85340
Patent: COMPOSITIONS ET MÉTHODES POUR TRAITER LE VIRUS DE L'HÉPATITE C (COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Patent: 50786
Patent: FORMULATION DE COMBINAISON DE DEUX COMPOSÉS ANTIVIRAUX (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 50013
Patent: FORMULATION DE COMBINAISON DE DEUX COMPOSÉS ANTIVIRAUX (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 77867
Patent: COMPOSITION ET PROCÉDÉS POUR LE TRAITEMENT DU VIRUS DE L'HÉPATITE C (COMPOSITION AND METHODS FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Germany
Patent: 2012013074
Estimated Expiration: ⤷ Get Started Free
Patent: 2012013382
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 02268
Patent: 用於治療 的組合物和方法 (COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 19869
Patent: 兩個抗病毒化合物的聯用製劑 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 31378
Patent: 用於治療 的組合物 (COMPOSITION FOR TREATING HCV HCV)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 36588
Estimated Expiration: ⤷ Get Started Free
Patent: 47777
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 1515
Patent: תכשירים לטיפול בוירוס הפטיטיס סי (Compositions for treating hcv)
Estimated Expiration: ⤷ Get Started Free
Patent: 2889
Patent: Gi7977 ושימושים שלו בטיפול בנגיף הפטיטיס c (Gi7977 and uses thereof in treating hepatitis c virus)
Estimated Expiration: ⤷ Get Started Free
Patent: 3419
Patent: פורמולציה משולבת של שתי תרכובות אנטי-ויראליות (Combination formulation of two antiviral compounds)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 99327
Estimated Expiration: ⤷ Get Started Free
Patent: 73897
Estimated Expiration: ⤷ Get Started Free
Patent: 23002
Estimated Expiration: ⤷ Get Started Free
Patent: 20994
Estimated Expiration: ⤷ Get Started Free
Patent: 14526516
Estimated Expiration: ⤷ Get Started Free
Patent: 14532657
Patent: C型肝炎ウイルスを処置するための方法および組成物
Estimated Expiration: ⤷ Get Started Free
Patent: 14533733
Patent: C型肝炎ウイルスを処置するための組成物および方法
Estimated Expiration: ⤷ Get Started Free
Patent: 15508418
Patent: 2種の抗ウイルス化合物のコンビナトリアル製剤
Estimated Expiration: ⤷ Get Started Free
Patent: 16053096
Patent: C型肝炎ウイルスを処置するための組成物および方法 (COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS)
Estimated Expiration: ⤷ Get Started Free
Patent: 16155875
Patent: 2種の抗ウイルス化合物のコンビナトリアル製剤 (COMBINATORIAL FORMULATION OF TWO KINDS OF ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 16166251
Patent: 2種の抗ウイルス化合物のコンビナトリアル製剤 (COMBINATORIAL FORMULATION OF TWO KINDS OF ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 17057230
Patent: HCVを処置するための方法 (METHOD FOR TREATING HCV)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 09613
Estimated Expiration: ⤷ Get Started Free
Patent: 50786
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 2166
Patent: COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 7735
Patent: COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6580
Patent: METODOS PARA EL TRATAMIENTO DE VHC. (METHODS FOR TREATING HCV.)
Estimated Expiration: ⤷ Get Started Free
Patent: 1816
Patent: FORMULACION DE COMBINACION DE DOS COMPUESTOS ANTIVIRALES. (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 4958
Patent: METODOS PARA EL TRATAMIENTO DE VHC. (METHODS FOR TREATING HCV.)
Estimated Expiration: ⤷ Get Started Free
Patent: 14003145
Patent: METODOS PARA EL TRATAMIENTO DE VHC. (METHODS FOR TREATING HCV.)
Estimated Expiration: ⤷ Get Started Free
Patent: 14005955
Patent: FORMULACION DE COMBINACION DE DOS COMPUESTOS ANTIVIRALES. (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS.)
Estimated Expiration: ⤷ Get Started Free
Patent: 14006373
Patent: COMPOSICIONES Y METODOS PARA TRATAR EL VIRUS DE LA HEPATITIS C. (COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS.)
Estimated Expiration: ⤷ Get Started Free
Moldova, Republic of
Patent: 30
Patent: Compoziţii şi metode de tratament al hepatitei virale C (Compositions and methods for treating hepatitis C virus)
Estimated Expiration: ⤷ Get Started Free
Patent: 89
Patent: Compoziţie farmaceutică cu conţinut de sofosbuvir şi utilizarea acesteia în tratamentul hepatitei virale C (Pharmaceutical composition comprising sofosbuvir and uses thereof for treating hepatitis C virus)
Estimated Expiration: ⤷ Get Started Free
Patent: 95
Patent: Tabletă combinată conţinând doi compuşi antivirali (Combination formulation of two antiviral compounds)
Estimated Expiration: ⤷ Get Started Free
Patent: 140035
Patent: Metodă de tratament al hepatitei virale C (Method for treating hepatitis C virus)
Estimated Expiration: ⤷ Get Started Free
Patent: 140058
Patent: Compoziţii şi metode de tratament al hepatitei virale C (Compositions and methods for treating hepatitis C virus)
Estimated Expiration: ⤷ Get Started Free
Patent: 150080
Patent: Compoziţie combinată de doi compuşi antivirali (Combination formulation of two antiviral compounds)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 009
Patent: METODE ZA LECENJE HCV-A (METHODS FOR TREATING HCV)
Estimated Expiration: ⤷ Get Started Free
Morocco
Patent: 906
Patent: Procédés permettant de traiter le virus de l'hépatite c (hcv)
Estimated Expiration: ⤷ Get Started Free
Patent: 150030
Patent: Compositions et méthodes pour traiter le virus de l'hépatite c
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 3396
Patent: Methods for treating hcv
Estimated Expiration: ⤷ Get Started Free
Patent: 5087
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 5532
Patent: Compositions and methods for treating hepatitis c virus
Estimated Expiration: ⤷ Get Started Free
Patent: 0391
Patent: Methods for treating hcv
Estimated Expiration: ⤷ Get Started Free
Patent: 9172
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 141056
Patent: METODOS PARA EL TRATAMIENTO DE VHC
Estimated Expiration: ⤷ Get Started Free
Patent: 141296
Patent: COMPOSICIONES Y METODOS PARA TRATAR EL VIRUS DE LA HEPATITIS C
Estimated Expiration: ⤷ Get Started Free
Patent: 151778
Patent: FORMULACION COMBINADA DE DOS COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014500557
Patent: METHODS FOR TREATING HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 014501133
Patent: COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS
Estimated Expiration: ⤷ Get Started Free
Patent: 015501710
Patent: COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 09613
Estimated Expiration: ⤷ Get Started Free
Patent: 50786
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 09613
Estimated Expiration: ⤷ Get Started Free
Patent: 50786
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01800087
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 975
Patent: METODE ZA LEČENJE HCV-A (METHODS FOR TREATING HCV)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201602044W
Patent: Methods For Treating HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 201706949V
Patent: COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 201400664W
Patent: METHODS FOR TREATING HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 201402609R
Patent: COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS
Estimated Expiration: ⤷ Get Started Free
Patent: 201506021X
Patent: COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 09613
Estimated Expiration: ⤷ Get Started Free
Patent: 50786
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1404061
Patent: COMPOSITIONS AND METHODS FOR TREATING THE HEPATITIS C VIRUS
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 1560994
Estimated Expiration: ⤷ Get Started Free
Patent: 1962522
Estimated Expiration: ⤷ Get Started Free
Patent: 1991298
Estimated Expiration: ⤷ Get Started Free
Patent: 140096029
Patent: METHODS FOR TREATING HCV
Estimated Expiration: ⤷ Get Started Free
Patent: 140108645
Patent: COMPOSITIONS AND METHODS FOR TREATING HEPATITIS C VIRUS
Estimated Expiration: ⤷ Get Started Free
Patent: 140119012
Patent: COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS
Estimated Expiration: ⤷ Get Started Free
Patent: 160052797
Patent: 두 항바이러스 화합물의 병용 제형물 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 190033643
Patent: 두 항바이러스 화합물의 병용 제형물 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Patent: 190075142
Patent: HCV 치료 방법 (HCV METHODS FOR TREATING HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 200060782
Patent: 두 항바이러스 화합물의 병용 제형물 (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 59216
Estimated Expiration: ⤷ Get Started Free
Patent: 71458
Estimated Expiration: ⤷ Get Started Free
Patent: 17886
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 66773
Estimated Expiration: ⤷ Get Started Free
Patent: 26047
Estimated Expiration: ⤷ Get Started Free
Patent: 1318627
Patent: Methods and compositions for treating hepatitis C virus
Estimated Expiration: ⤷ Get Started Free
Patent: 1325599
Patent: Compositions and methods for treating hepatitis C virus
Estimated Expiration: ⤷ Get Started Free
Patent: 1444556
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 1840311
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Patent: 2042808
Patent: Combination formulation of two antiviral compounds
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1802537
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 4097
Patent: КОМПОЗИЦІЯ (ВАРІАНТИ) ТА СПОСІБ (ВАРІАНТИ) ЛІКУВАННЯ ВІРУСНОГО ГЕПАТИТУ С
Estimated Expiration: ⤷ Get Started Free
Patent: 6087
Patent: КОМПОЗИЦІЯ ДЛЯ ЛІКУВАННЯ ВІРУСУ ГЕПАТИТУ C (METHODS FOR TREATING HCV)
Estimated Expiration: ⤷ Get Started Free
Patent: 8256
Patent: КОМБІНОВАНИЙ СКЛАД ДВОХ ПРОТИВІРУСНИХ СПОЛУК (COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS)
Estimated Expiration: ⤷ Get Started Free
Uruguay
Patent: 420
Patent: ?MÉTODOS DE TRATAMIENTO DEL VIRUS DE LA HEPATITIS C CON QUE DE GS-7977 Y RIBAVIRINA, COMPOSICIONES Y USOS"
Estimated Expiration: ⤷ Get Started Free
Patent: 474
Patent: Composiciones, forma de dosificación y métodos para tratar el virus de la hepatitis C.
Estimated Expiration: ⤷ Get Started Free
Patent: 299
Patent: FORMULACIÓN COMBINADA DE DOS COMPUESTOS ANTIVIRALES
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering HARVONI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2752422 | ⤷ Get Started Free | |
| Japan | 2014148550 | ANTIVIRAL COMPOUND | ⤷ Get Started Free |
| Malaysia | 172166 | COMBINATION FORMULATION OF TWO ANTIVIRAL COMPOUNDS | ⤷ Get Started Free |
| Australia | 2012346217 | ⤷ Get Started Free | |
| Peru | 20141296 | ⤷ Get Started Free | |
| Croatia | P20140667 | ⤷ Get Started Free | |
| Japan | 5872539 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for HARVONI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2203462 | 1490066-6 | Sweden | ⤷ Get Started Free | PRODUCT NAME: SOFOSBUVIR |
| 2430014 | C201630002 | Spain | ⤷ Get Started Free | PRODUCT NAME: LEDIPASVIR; NATIONAL AUTHORISATION NUMBER: EU/1/14/958; DATE OF AUTHORISATION: 20141117; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/14/958; DATE OF FIRST AUTHORISATION IN EEA: 20141117 |
| 2203462 | 1491066-5 | Sweden | ⤷ Get Started Free | PRODUCT NAME: SOFOSBUVIR; FIRST MARKETING AUTHORIZATION NUMBER SE: EG EU/1/13/894, 2014-01-17 |
| 2430014 | 16C0005 | France | ⤷ Get Started Free | PRODUCT NAME: LEDIPASVIR; REGISTRATION NO/DATE: EU/1/14/958/001-002 20141118 |
| 2430014 | 2016C/006 | Belgium | ⤷ Get Started Free | PRODUCT NAME: LEDIPASVIR/SOFOSBUVIR; AUTHORISATION NUMBER AND DATE: EU/1/14/958 20141118 |
| 2430014 | CA 2016 00006 | Denmark | ⤷ Get Started Free | PRODUCT NAME: LEDISPAVIR; REG. NO/DATE: EU/1/14/958/001-002 20141118 |
| 2203462 | PA2014040,C2203462 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: SOFOSBUVIRAS; REGISTRATION NO/DATE: EU/1/13/894/001 - EU/1/13/894/002 20140116 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for HARVONI
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
