Last updated: July 28, 2025
Introduction
Fenofibric acid, a lipid-modifying agent primarily used to reduce triglycerides and increase HDL cholesterol, has emerged as a critical asset within the cardiovascular therapeutic landscape. As a metabolite of fenofibrate, fenofibric acid offers enhanced bioavailability and potency, positioning it as a preferred choice in dyslipidemia management. Analyzing its market dynamics and financial trajectory involves evaluating regulatory pathways, competitive positioning, commercialization strategies, and emerging market trends that drive its adoption.
Regulatory Environment and Market Entry
The regulatory pathway significantly influences the financial outlook of fenofibric acid. In the United States, the FDA approved the branded drug Trilipix (fenofibric acid) in 2007, emphasizing its differentiated formulation. The approval process was characterized by rigorous clinical evidence demonstrating non-inferiority or superiority over fenofibrate, reinforcing its market position. Notably, recent shifts in regulatory priorities, including increased scrutiny over cardiovascular outcomes, necessitate continuous post-market studies to sustain approval and market access.
In Europe, fenofibric acid's regulatory pathway follows the centralized EMA procedures, with approval contingent on comprehensive safety and efficacy data. The evolving landscape emphasizes aligning formulations with clinical guidelines and demonstrating cardiovascular benefit—factors that influence the drug's financial prospects.
Market Demand and Therapeutic Trends
The global prevalence of dyslipidemia, compounded by rising rates of cardiovascular disease (CVD), underpins sustained demand for lipid-lowering agents. Market research indicates that the global hyperlipidemia market is projected to grow at a CAGR of approximately 5-7% through 2028[1]. Fenofibric acid benefits from this trend owing to its efficacy in triglyceride reduction and HDL elevation—key therapeutic endpoints supported by clinical guidelines from the American Association of Clinical Endocrinologists and the American College of Cardiology.
Moreover, the shift toward combination therapy—pairing fenofibric acid with statins—is reshaping prescribing patterns. The ability to address residual cardiovascular risk further enhances its clinical utility. Additionally, the increasing focus on managing metabolic syndrome and non-alcoholic fatty liver disease (NAFLD) elevates the relevance of fenofibric acid, expanding its target market.
Competitive Landscape and Market Positioning
Fenofibric acid faces competition from several generic and branded formulations. Generic fenofibrate remains a cost-effective alternative, especially in regions with high price sensitivity. However, branded fenofibric acid formulations, such as Trilipix, offer advantages like improved tolerability and pharmacokinetic profiles, which appeal to prescribers seeking optimized patient outcomes.
Emerging competitors include novel CETP inhibitors and PCSK9 inhibitors, though their applications are more targeted toward LDL reduction rather than triglycerides. The differentiation of fenofibric acid in terms of safety profile, tolerability, and dosing convenience sustains its market share. Strategic partnerships and licensing agreements play a vital role in expanding its footprint, particularly in emerging markets where regulatory and reimbursement pathways are evolving.
Manufacturing and Supply Chain Considerations
Manufacturing complexities influence the device's gross margins and financial performance. Fenofibric acid production involves stringent quality controls due to strict regulatory standards. The shift toward biosimilar and generic versions in mature markets introduces pricing pressures, compelling manufacturers to optimize supply chains and reduce costs.
Sourcing raw materials, such as fenofibrate intermediates, and ensuring compliance with Good Manufacturing Practices (GMP) are critical in maintaining product quality while managing manufacturing expenses. Additionally, patent expirations or challenge periods can influence market exclusivity, impacting revenue streams.
Pricing Strategies and Reimbursement Dynamics
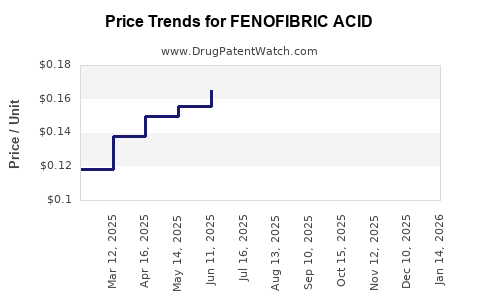
Pricing strategies for fenofibric acid align closely with market competition, patent protection, and reimbursement policies. Branded formulations command premium pricing supported by clinical benefits, while generics thrive on price sensitivity. In markets with robust reimbursement frameworks, higher-priced branded drugs sustain profitability.
Reimbursement policies are increasingly tied to demonstrated cardiovascular outcomes, compelling manufacturers to invest in post-market studies. Payers' shifting preferences toward cost-effective therapies influence formulary decisions, affecting sales volume and revenue.
Emerging Market Opportunities
The rising prevalence of dyslipidemia in Asia-Pacific and Latin America presents substantial opportunities. Market penetration strategies include localized clinical trials, partnerships with regional distributors, and adaptation to regional regulatory requirements. The expanding healthcare infrastructure facilitates access to fenofibric acid, although pricing and reimbursement landscape disparities pose challenges.
Furthermore, biosimilar development and efforts to extend patent exclusivity through patent extensions or supplementary protection certificates can prolong market life and enhance revenue prospects.
Financial Trajectory and Future Outlook
The financial trajectory for fenofibric acid appears positive, leveraging global market growth, clinical demand, and strategic positioning. Analysts project a compound annual growth rate (CAGR) of approximately 4-6% over the next five years, driven by increasing cardiovascular disease burden and expanding indications[2].
In the near term, revenue growth is anticipated through increased adoption in combination therapy regimens and expansion into emerging markets. Long-term growth hinges on sustaining regulatory approval, navigating patent landscapes, and integrating innovative delivery approaches, such as controlled-release formulations.
Research and development investments aimed at demonstrating superior cardiovascular endpoints or exploring new indications (e.g., NAFLD) could augment profitability. Conversely, patent expirations pose a risk for revenue erosion, emphasizing the need for lifecycle management strategies.
Conclusion
Fenofibric acid's market dynamics are shaped by a confluence of regulatory frameworks, clinical demand, competitive positioning, and emerging market opportunities. Its financial trajectory remains promising, supported by demographic trends and clinical utility, though vulnerable to patent cliffs and pricing pressures. Strategic collaborations, continual clinical evidence generation, and geographic expansion are vital for maximizing its commercial potential.
Key Takeaways
- Growing Market Need: Rising dyslipidemia and CVD prevalence globally sustain demand for fenofibric acid.
- Regulatory & Clinical Validation: Favorable regulatory approvals and clinical efficacy reinforce market presence.
- Competitive & Pricing Strategies: Balance between branded premium and generic affordability influences revenue streams.
- Emerging Market Expansion: Significant growth opportunities in Asia-Pacific and developing regions.
- Lifecycle Management: Patent expirations and competitive landscape necessitate innovation and strategic partnerships.
FAQs
-
What distinguishes fenofibric acid from fenofibrate in the market?
Fenofibric acid offers improved bioavailability and tolerability, resulting in enhanced efficacy for triglyceride reduction and HDL increase.
-
How do regulatory agencies impact fenofibric acid’s market potential?
Regulatory approvals, especially demonstrations of cardiovascular benefits, validate its clinical utility and influence reimbursement and market access.
-
What are the main competitive challenges facing fenofibric acid?
Generic fenofibrate availability, emerging lipid-lowering therapies, and patent expirations challenge its market share.
-
What growth opportunities exist in emerging markets?
Expanding healthcare infrastructure, increasing dyslipidemia prevalence, and unmet medical needs present substantial expansion prospects.
-
How does patent protection influence fenofibric acid’s financial outlook?
Patent protections enable premium pricing and exclusivity; expirations lead to generic price erosion but open avenues for biosimilar competition and lifecycle extensions.
Sources:
[1] Global Hyperlipidemia Market Report, 2022-2028
[2] Market Analysis on Lipid-Lowering Agents, 2023