ERLEADA Drug Patent Profile
✉ Email this page to a colleague
When do Erleada patents expire, and when can generic versions of Erleada launch?
Erleada is a drug marketed by Janssen Biotech and is included in one NDA. There are twelve patents protecting this drug and two Paragraph IV challenges.
This drug has three hundred and fifty patent family members in forty-nine countries.
The generic ingredient in ERLEADA is apalutamide. One supplier is listed for this compound. Additional details are available on the apalutamide profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Erleada
A generic version of ERLEADA was approved as apalutamide by ZYDUS on March 17th, 2025.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ERLEADA?
- What are the global sales for ERLEADA?
- What is Average Wholesale Price for ERLEADA?
Summary for ERLEADA
| International Patents: | 350 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 74 |
| Clinical Trials: | 22 |
| Patent Applications: | 1,693 |
| Drug Prices: | Drug price information for ERLEADA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ERLEADA |
| What excipients (inactive ingredients) are in ERLEADA? | ERLEADA excipients list |
| DailyMed Link: | ERLEADA at DailyMed |

Recent Clinical Trials for ERLEADA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Washington | Phase 2 |
| Jonsson Comprehensive Cancer Center | Phase 2 |
| National Cancer Institute (NCI) | Phase 1 |
Paragraph IV (Patent) Challenges for ERLEADA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ERLEADA | Tablets | apalutamide | 240 mg | 210951 | 1 | 2025-03-20 |
| ERLEADA | Tablets | apalutamide | 60 mg | 210951 | 5 | 2022-02-14 |
US Patents and Regulatory Information for ERLEADA
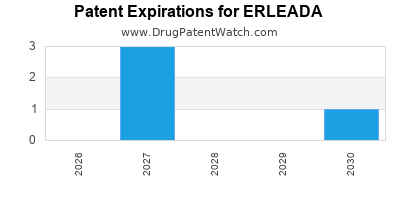
ERLEADA is protected by sixteen US patents.
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Biotech | ERLEADA | apalutamide | TABLET;ORAL | 210951-002 | Feb 17, 2023 | RX | Yes | Yes | 12,303,493 | ⤷ Get Started Free | Y | ⤷ Get Started Free | |||
| Janssen Biotech | ERLEADA | apalutamide | TABLET;ORAL | 210951-002 | Feb 17, 2023 | RX | Yes | Yes | 9,481,663 | ⤷ Get Started Free | Y | Y | ⤷ Get Started Free | ||
| Janssen Biotech | ERLEADA | apalutamide | TABLET;ORAL | 210951-001 | Feb 14, 2018 | AB | RX | Yes | No | 8,802,689 | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ERLEADA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International NV | Erleada | apalutamide | EMEA/H/C/004452Erleada is indicated:in adult men for the treatment of non metastatic castration resistant prostate cancer (nmCRPC) who are at high risk of developing metastatic disease.in adult men for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (ADT). | Authorised | no | no | no | 2019-01-14 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ERLEADA
When does loss-of-exclusivity occur for ERLEADA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 13271751
Patent: Crystalline forms of an androgen receptor modulator
Estimated Expiration: ⤷ Get Started Free
Patent: 17200298
Patent: Crystalline forms of an androgen receptor modulator
Estimated Expiration: ⤷ Get Started Free
Patent: 17279807
Estimated Expiration: ⤷ Get Started Free
Brazil
Patent: 2014030678
Patent: formas cristalinas de um modulador de receptor de androgênio
Estimated Expiration: ⤷ Get Started Free
Canada
Patent: 75767
Patent: FORMES CRISTALLINES D'UN MODULATEUR DU RECEPTEUR DES ANDROGENES (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 08345
Patent: FORMES CRISTALLINES D'UN MODULATEUR DE RECEPTEUR D'ANDROGENE (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 55660
Patent: FORMES CRISTALLINES D'UN MODULATEUR DE RECEPTEUR D'ANDROGENE (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 14726
Patent: FORMES CRISTALLINES D'UN MODULATEUR DE RECEPTEUR D'ANDROGENE (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Chile
Patent: 14003331
Patent: Formas cristalinas de un modulador de receptor de androgeno
Estimated Expiration: ⤷ Get Started Free
China
Patent: 4619692
Patent: Crystalline forms of an androgen receptor modulator
Estimated Expiration: ⤷ Get Started Free
Patent: 5693692
Patent: 雄激素受体调节剂的晶形
Estimated Expiration: ⤷ Get Started Free
Patent: 3135892
Patent: 雄激素受体调节剂的晶形 (Crystalline form of androgen receptor modulator)
Estimated Expiration: ⤷ Get Started Free
Colombia
Patent: 40407
Patent: Formas cristalinas de un modulador del receptor androgénico
Estimated Expiration: ⤷ Get Started Free
Costa Rica
Patent: 140549
Patent: FORMAS CRISTALINAS DE UN MODULADOR DEL RECEPTOR ANDROGÉNICO
Estimated Expiration: ⤷ Get Started Free
Patent: 190331
Patent: UNA FORMA CRISTALINA A DE UN MODULADOR DEL RECEPTOR ANDROGÉNICO (Divisional 2014-0549) (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Croatia
Patent: 0180902
Estimated Expiration: ⤷ Get Started Free
Patent: 0201387
Estimated Expiration: ⤷ Get Started Free
Patent: 0210909
Estimated Expiration: ⤷ Get Started Free
Cyprus
Patent: 20393
Estimated Expiration: ⤷ Get Started Free
Patent: 23427
Estimated Expiration: ⤷ Get Started Free
Patent: 24831
Estimated Expiration: ⤷ Get Started Free
Patent: 21032
Estimated Expiration: ⤷ Get Started Free
Denmark
Patent: 58985
Estimated Expiration: ⤷ Get Started Free
Patent: 48553
Estimated Expiration: ⤷ Get Started Free
Patent: 33792
Estimated Expiration: ⤷ Get Started Free
Ecuador
Patent: 14030098
Patent: FORMAS CRISTALINAS DE UN MODULADOR DEL RECEPTOR ANDROGÉNICO
Estimated Expiration: ⤷ Get Started Free
Eurasian Patent Organization
Patent: 8791
Patent: КРИСТАЛЛИЧЕСКАЯ ФОРМА МОДУЛЯТОРА АНДРОГЕННЫХ РЕЦЕПТОРОВ (CRYSTALLINE FORM OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 3956
Patent: КРИСТАЛЛИЧЕСКАЯ ФОРМА А 4-[7-(6-ЦИАНО-5-ТРИФТОРМЕТИЛПИРИДИН-3-ИЛ)-8-ОКСО-6-ТИОКСО-5,7- ДИАЗАСПИРО[3.4]ОКТ-5-ИЛ]-2-ФТОР-N-МЕТИЛБЕНЗАМИДА ДЛЯ ЛЕЧЕНИЯ ЗАБОЛЕВАНИЯ ИЛИ СОСТОЯНИЯ, СВЯЗАННОГО С АКТИВНОСТЬЮ АНДРОГЕННЫХ РЕЦЕПТОРОВ, ВКЛЮЧАЯ РАК ПРОСТАТЫ (CRYSTALLINE FORM OF 4-[7-(6-CYANO-5-TRIFLUOROMETHYLPYRIDIN-3-YL)-8-OXO-6-THIOXO-5,7-DIAZASPIRO[3.4]OCT-5-YL]-2-FLUORO-N-METHYLBENZAMIDE FOR TREATING A DISEASE OR CONDITION ASSOCIATED WITH ANDROGEN RECEPTOR ACTIVITY, INCLUDING PROSTATE CANCER)
Estimated Expiration: ⤷ Get Started Free
Patent: 1492272
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ МОДУЛЯТОРА АНДРОГЕННЫХ РЕЦЕПТОРОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 1791592
Patent: КРИСТАЛЛИЧЕСКИЕ ФОРМЫ МОДУЛЯТОРА АНДРОГЕННЫХ РЕЦЕПТОРОВ
Estimated Expiration: ⤷ Get Started Free
Patent: 1992010
Patent: КРИСТАЛЛИЧЕСКАЯ ФОРМА А 4-[7-(6-ЦИАНО-5-ТРИФТОРМЕТИЛПИРИДИН-3-ИЛ)-8-ОКСО-6-ТИОКСО-5,7-ДИАЗАСПИРО[3.4]ОКТ-5-ИЛ]-2-ФТОР-N-МЕТИЛБЕНЗАМИДА ДЛЯ ЛЕЧЕНИЯ ЗАБОЛЕВАНИЯ ИЛИ СОСТОЯНИЯ, СВЯЗАННОГО С АКТИВНОСТЬЮ АНДРОГЕННЫХ РЕЦЕПТОРОВ, ВКЛЮЧАЯ РАК ПРОСТАТЫ
Estimated Expiration: ⤷ Get Started Free
European Patent Office
Patent: 58985
Patent: FORMES CRISTALLINES D'UN MODULATEUR DU RÉCEPTEUR DES ANDROGÈNES (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 48553
Patent: FORMES CRISTALLINES D'UN MODULATEUR DE RÉCEPTEUR D'ANDROGÈNE (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 33792
Patent: FORMES CRISTALLINES D'UN MODULATEUR DE RÉCEPTEUR D'ANDROGÈNE (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 22629
Patent: FORMES CRISTALLINES D'UN MODULATEUR DE RÉCEPTEUR D'ANDROGÈNE (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
France
Patent: C1050
Estimated Expiration: ⤷ Get Started Free
Guatemala
Patent: 1400283
Patent: FORMAS CRISTALINAS DE UN MODULADOR DEL RECEPTOR ANDROGÉNICO
Estimated Expiration: ⤷ Get Started Free
Hong Kong
Patent: 10175
Patent: 雄激素受體調節劑的晶形 (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 26066
Patent: 雄激素受體調節劑的晶形 (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Hungary
Patent: 38082
Estimated Expiration: ⤷ Get Started Free
Patent: 50357
Estimated Expiration: ⤷ Get Started Free
Patent: 54595
Estimated Expiration: ⤷ Get Started Free
Patent: 100047
Estimated Expiration: ⤷ Get Started Free
India
Patent: 084DEN2014
Estimated Expiration: ⤷ Get Started Free
Israel
Patent: 9738
Patent: צורות גבישיות של מאפנן קולטן אנדרוגן (Crystalline forms of an androgen receptor modulator)
Estimated Expiration: ⤷ Get Started Free
Patent: 7608
Patent: צורות גבישיות של מאפנן קולטן אנדרוגן (Crystalline forms of an androgen receptor modulator)
Estimated Expiration: ⤷ Get Started Free
Patent: 5413
Patent: צורות גבישיות של מאפנן קולטן אנדרוגן (Crystalline forms of an androgen receptor modulator)
Estimated Expiration: ⤷ Get Started Free
Patent: 0522
Patent: צורות גבישיות של מאפנן קולטן אנדרוגן (Crystalline forms of an androgen receptor modulator)
Estimated Expiration: ⤷ Get Started Free
Japan
Patent: 82209
Estimated Expiration: ⤷ Get Started Free
Patent: 45821
Estimated Expiration: ⤷ Get Started Free
Patent: 15518890
Patent: アンドロゲン受容体変調剤の結晶質形態
Estimated Expiration: ⤷ Get Started Free
Patent: 17178923
Patent: アンドロゲン受容体変調剤の結晶質形態 (CRYSTALLINE FORMS OF ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 18141009
Patent: アンドロゲン受容体変調剤の結晶質形態 (CRYSTALLINE FORMS OF ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Lithuania
Patent: 533792
Estimated Expiration: ⤷ Get Started Free
Patent: 2021525
Estimated Expiration: ⤷ Get Started Free
Patent: 58985
Estimated Expiration: ⤷ Get Started Free
Patent: 48553
Estimated Expiration: ⤷ Get Started Free
Patent: 33792
Estimated Expiration: ⤷ Get Started Free
Luxembourg
Patent: 0236
Estimated Expiration: ⤷ Get Started Free
Malaysia
Patent: 7500
Patent: CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
Mexico
Patent: 6754
Patent: FORMAS CRISTALINAS DE UN MODULADOR DEL RECEPTOR ANDROGENICO. (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR.)
Estimated Expiration: ⤷ Get Started Free
Patent: 14015005
Patent: FORMAS CRISTALINAS DE UN MODULADOR DEL RECEPTOR ANDROGENICO. (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR.)
Estimated Expiration: ⤷ Get Started Free
Montenegro
Patent: 081
Patent: MODULATORA RECEPTORA ANDROGENA PATENTNI ZAHTEVI (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 815
Patent: KRISTALNI OBLICI MODULATORA RECEPTORA ANDROGENA (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Netherlands
Patent: 1144
Estimated Expiration: ⤷ Get Started Free
New Zealand
Patent: 2203
Patent: Crystalline forms of an androgen receptor modulator
Estimated Expiration: ⤷ Get Started Free
Patent: 7683
Patent: Crystalline forms of an androgen receptor modulator
Estimated Expiration: ⤷ Get Started Free
Nicaragua
Patent: 1400142
Patent: FORMAS CRISTALINAS DE UN MODULADOR DEL RECEPTOR DE ANDROGENOS
Estimated Expiration: ⤷ Get Started Free
Norway
Patent: 21046
Estimated Expiration: ⤷ Get Started Free
Peru
Patent: 150631
Patent: FORMAS CRISTALINAS DE UN MODULADOR DEL RECEPTOR ANDROGENICO
Estimated Expiration: ⤷ Get Started Free
Patent: 200725
Patent: FORMAS CRISTALINAS DEL MODULADOR RECEPTOR DE ANDROGENO: 4-[7-(6-CIANO-5-TRIFLUOROMETILPIRIDIN-3-IL)-8-OXO-6-TIOXO-5,7-DIAZAESPIRO[3,4]OCT-5-IL]-2-FLUORO-N-METILBENZAMIDA) (APALUTAMIDA)
Estimated Expiration: ⤷ Get Started Free
Patent: 200795
Patent: COMPOSICION QUE COMPRENDE UNA FORMA CRISTALINA DEL MODULADOR RECEPTOR DE ANDROGENO: 4-[7-(6-CIANO-5-TRIFLUOMETILPIRIDIN-3-IL)-8-OXO-6-TIOXO-5,7-DIAZAESPIRO [3.4]OCT-5-IL]-2-FLUORO-N-METILBENZAMIDA (APALUTAMIDA)
Estimated Expiration: ⤷ Get Started Free
Philippines
Patent: 014502714
Patent: CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
Patent: 016501470
Patent: CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
Poland
Patent: 58985
Estimated Expiration: ⤷ Get Started Free
Patent: 48553
Estimated Expiration: ⤷ Get Started Free
Patent: 33792
Estimated Expiration: ⤷ Get Started Free
Portugal
Patent: 58985
Estimated Expiration: ⤷ Get Started Free
Patent: 48553
Estimated Expiration: ⤷ Get Started Free
Patent: 33792
Estimated Expiration: ⤷ Get Started Free
San Marino
Patent: 01800311
Estimated Expiration: ⤷ Get Started Free
Patent: 02000496
Estimated Expiration: ⤷ Get Started Free
Patent: 02100355
Estimated Expiration: ⤷ Get Started Free
Serbia
Patent: 370
Patent: KRISTALNI OBLICI MODULATORA RECEPTORA ANDROGENA (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 617
Patent: KRISTALNI OBLICI MODULATORA RECEPTORA ANDROGENA (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 988
Patent: KRISTALNI OBLICI MODULATORA RECEPTORA ANDROGENA (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Singapore
Patent: 201610248S
Patent: CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
Patent: 201610249T
Patent: CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
Patent: 201408140Q
Patent: CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
Slovenia
Patent: 58985
Estimated Expiration: ⤷ Get Started Free
Patent: 48553
Estimated Expiration: ⤷ Get Started Free
Patent: 33792
Estimated Expiration: ⤷ Get Started Free
South Africa
Patent: 1500076
Patent: CRYSTALLINE FORMS OF ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
South Korea
Patent: 2062024
Estimated Expiration: ⤷ Get Started Free
Patent: 2195916
Estimated Expiration: ⤷ Get Started Free
Patent: 150021993
Patent: CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR
Estimated Expiration: ⤷ Get Started Free
Patent: 190132543
Patent: 안드로겐 수용체 조절제의 결정 형태 (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Spain
Patent: 70683
Estimated Expiration: ⤷ Get Started Free
Patent: 09738
Estimated Expiration: ⤷ Get Started Free
Patent: 75932
Estimated Expiration: ⤷ Get Started Free
Taiwan
Patent: 32732
Estimated Expiration: ⤷ Get Started Free
Patent: 1402561
Patent: Crystalline forms of an androgen receptor modulator
Estimated Expiration: ⤷ Get Started Free
Turkey
Patent: 1808939
Estimated Expiration: ⤷ Get Started Free
Ukraine
Patent: 5665
Patent: КРИСТАЛІЧНІ ФОРМИ МОДУЛЯТОРА АНДРОГЕННОГО РЕЦЕПТОРА (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR)
Estimated Expiration: ⤷ Get Started Free
Patent: 3142
Patent: КРИСТАЛІЧНІ ФОРМИ МОДУЛЯТОРА АНДРОГЕННОГО РЕЦЕПТОРА
Estimated Expiration: ⤷ Get Started Free
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ERLEADA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Canada | 3008345 | FORMES CRISTALLINES D'UN MODULATEUR DE RECEPTEUR D'ANDROGENE (CRYSTALLINE FORMS OF AN ANDROGEN RECEPTOR MODULATOR) | ⤷ Get Started Free |
| Japan | 6701237 | ⤷ Get Started Free | |
| Lithuania | 3412290 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ERLEADA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3533792 | LUC00236 | Luxembourg | ⤷ Get Started Free | PRODUCT NAME: APALUTAMIDE; AUTHORISATION NUMBER AND DATE: EU/1/18/1342 20190116 |
| 3533792 | 122021000067 | Germany | ⤷ Get Started Free | PRODUCT NAME: APALUTAMID; REGISTRATION NO/DATE: EU/1/18/1342 20190114 |
| 3533792 | CA 2021 00041 | Denmark | ⤷ Get Started Free | PRODUCT NAME: APALUTAMID; REG. NO/DATE: EU/1/18/1342 20190116 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for the Pharmaceutical Drug: ERLEADA
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
