EPRONTIA Drug Patent Profile
✉ Email this page to a colleague
When do Eprontia patents expire, and when can generic versions of Eprontia launch?
Eprontia is a drug marketed by Azurity and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
The generic ingredient in EPRONTIA is topiramate. There are twenty-six drug master file entries for this compound. Fifty-three suppliers are listed for this compound. Additional details are available on the topiramate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Eprontia
A generic version of EPRONTIA was approved as topiramate by ACCORD HLTHCARE on March 27th, 2009.
AI Research Assistant
Questions you can ask:
- What is the 5 year forecast for EPRONTIA?
- What are the global sales for EPRONTIA?
- What is Average Wholesale Price for EPRONTIA?
Summary for EPRONTIA
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 103 |
| Patent Applications: | 5,077 |
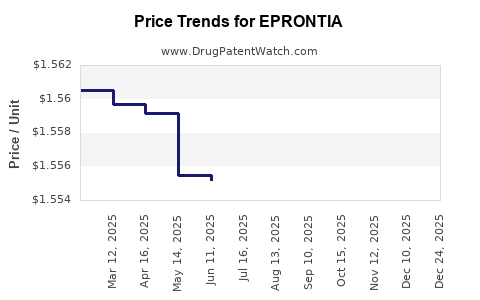
| Drug Prices: | Drug price information for EPRONTIA |
| What excipients (inactive ingredients) are in EPRONTIA? | EPRONTIA excipients list |
| DailyMed Link: | EPRONTIA at DailyMed |
Pharmacology for EPRONTIA
| Mechanism of Action | Cytochrome P450 2C19 Inhibitors Cytochrome P450 3A4 Inducers |
| Physiological Effect | Decreased Central Nervous System Disorganized Electrical Activity |
Paragraph IV (Patent) Challenges for EPRONTIA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| EPRONTIA | Oral Solution | topiramate | 25 mg/mL | 214679 | 1 | 2022-10-06 |
US Patents and Regulatory Information for EPRONTIA
EPRONTIA is protected by eight US patents.
Patents protecting EPRONTIA
Compositions and methods for treating epilepsy, seizures and other conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED AS ADJUNCTIVE THERAPY FOR THE TREATMENT OF PARTIAL-ONSET SEIZURES, PRIMARY GENERALIZED TONIC-CLONIC SEIZURES, AND SEIZURES ASSOCIATED WITH LENNOX-GASTAUT SYNDROME IN PATIENTS 2 YEARS OF AGE AND OLDER
Compositions and methods for treating epilepsy, seizures and other conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED AS INITIAL MONOTHERAPY FOR THE TREATMENT OF PARTIAL-ONSET OR PRIMARY GENERALIZED TONIC-CLONIC SEIZURES IN PATIENTS 2 YEARS OF AGE AND OLDER
Compositions and methods for treating epilepsy, seizures and other conditions
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED FOR THE PREVENTIVE TREATMENT OF MIGRAINE IN PATIENTS 12 YEARS AND OLDER
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED AS ADJUNCTIVE THERAPY FOR THE TREATMENT OF PARTIAL-ONSET SEIZURES, PRIMARY GENERALIZED TONIC-CLONIC SEIZURES, AND SEIZURES ASSOCIATED WITH LENNOX-GASTAUT SYNDROME IN PATIENTS 2 YEARS OF AGE AND OLDER
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED AS INITIAL MONOTHERAPY FOR THE TREATMENT OF PARTIAL-ONSET OR PRIMARY GENERALIZED TONIC-CLONIC SEIZURES IN PATIENTS 2 YEARS OF AGE AND OLDER
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: INDICATED FOR THE PREVENTIVE TREATMENT OF MIGRAINE IN PATIENTS 12 YEARS AND OLDER
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azurity | EPRONTIA | topiramate | SOLUTION;ORAL | 214679-001 | Nov 5, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Azurity | EPRONTIA | topiramate | SOLUTION;ORAL | 214679-001 | Nov 5, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Azurity | EPRONTIA | topiramate | SOLUTION;ORAL | 214679-001 | Nov 5, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Azurity | EPRONTIA | topiramate | SOLUTION;ORAL | 214679-001 | Nov 5, 2021 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |


