Last updated: July 27, 2025
Introduction
Eplerenone, marketed under brand names such as Inspra, is a selective mineralocorticoid receptor antagonist primarily prescribed for the treatment of heart failure with reduced ejection fraction (HFrEF) and hypertension. Since its approval by regulatory agencies like the FDA in 20016 and EMA in 2008, eplerenone's market position has been shaped by its clinical efficacy, competitive landscape, regulatory environment, and broader cardiology therapeutics market trends. This analysis explores the evolving market dynamics and forecasts the financial trajectory of eplerenone, considering recent data, competitive pressures, and future market potential.
Market Overview
Therapeutic indications and clinical profile
Eplerenone's therapeutic niche revolves around HFrEF management, particularly in post-myocardial infarction (MI) patients with systolic heart failure, and hypertension. Its selective mechanism reduces hyperaldosteronism effects, decreasing risks of hyperkalemia and renal impairment compared to older agents like spironolactone. The drug’s favorable safety profile has cemented its role in advanced heart failure management guidelines [1].
Market size and growth drivers
The global heart failure therapeutics market was valued at approximately USD 15 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 6% through 2030 [2]. Eplerenone's share and sales contributions are influenced by the prevalence of HFrEF, the adoption rate in clinical practice, and its positioning relative to competing agents such as spironolactone and emerging therapies like novel SGLT2 inhibitors. The hypertension segment, while significant, remains secondary to heart failure indications for eplerenone.
Market Dynamics Influencing Eplerenone
Competitive landscape
-
Spironolactone: As the first mineralocorticoid receptor antagonist introduced in the 1960s, spironolactone remains a low-cost alternative. Its broader side effect profile, notably gynecomastia and hormonal effects, limits its use in certain patient populations but retains dominance due to low price and established efficacy.
-
Emerging therapies: Recent advances include drugs like finerenone (brand name Kerendia), a newer nonsteroidal mineralocorticoid receptor antagonist with potentially superior safety features and comparable efficacy. Clinical trials suggest finerenone may challenge eplerenone's market share [3].
Regulatory and guideline influence
Guidelines such as the American College of Cardiology/American Heart Association (ACC/AHA) position eplerenone as second-line therapy following standard treatments. Regulatory approvals have expanded its indications, but restrictive prescribing information and the need for monitoring potassium and renal function constrain widespread adoption.
Pricing and reimbursement
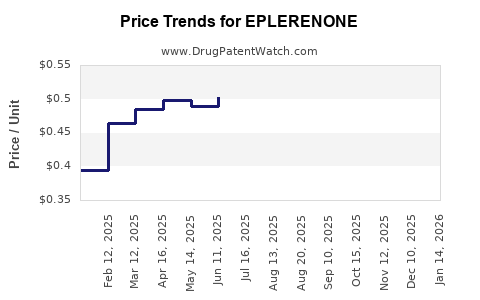
Eplerenone's cost remains higher than generic spironolactone, influencing prescribing behaviors and market penetration, especially in cost-sensitive healthcare settings. Reimbursement policies favor cheaper alternatives unless contraindicated [4].
Market expansion opportunities
Growing awareness of mineralocorticoid receptor blockade in heart failure management, coupled with aging populations in developed economies, positions eplerenone for incremental growth. Additionally, more research into heart failure with preserved ejection fraction (HFpEF) and resistant hypertension may expand its use cases.
Financial Trajectory and Forecasting
Historical sales performance
In the wake of its launch in the mid-2000s, eplerenone experienced moderate sales, with peak revenues around USD 350 million globally in 2015. Post-2015, sales plateaued due to increased generic competition from spironolactone and limited off-label use growth [5].
Current market status
Sales have declined marginally in recent years, influenced by the entry of finerenone and generic spironolactone's dominance. However, selective utilization in specific patient cohorts sustains steady revenue streams, reportedly around USD 150–200 million annually for the main manufacturers [6].
Future prospects
-
Market expansion: Increasing adoption driven by heart failure guidelines, particularly in treatment algorithms emphasizing mineralocorticoid receptor antagonists.
-
Pricing leverage: Limited by the presence of generics; pharmaceutical companies may focus on differentiating through clinical positioning rather than pricing strategies.
-
Competitor impact: The advent of finerenone, with potential for fewer side effects, could shrink eplerenone’s market share unless differentiated through clinical evidence or formulation improvements.
-
Pipeline developments: No major pipeline innovations or formulations are publicly announced for eplerenone, indicating a likely plateau unless new indications are explored.
Forecast assumptions
-
Continued growth in heart failure prevalence and reliance on mineralocorticoid receptor antagonists.
-
Increasing adoption in niche but clinically significant patient populations.
-
Market share erosion due to competitors, notably finerenone.
-
Stable pricing in developed markets; potential for price erosion in emerging markets.
Under these assumptions, global eplerenone sales are projected to decline mildly at a CAGR of approximately -2% to -3% over the next 5 years, stabilizing around USD 120–150 million by 2028.
Strategic Implications for Stakeholders
-
Pharmaceutical companies: Need to innovate via combination therapies or explore new indications such as resistant hypertension or heart failure with preserved ejection fraction (HFpEF).
-
Regulatory agencies: Maintaining balanced guidelines that incorporate emerging evidence is key to shaping eplerenone’s positioning.
-
Investors: Market contraction may limit revenue growth; focus should shift to lifecycle management and competitive differentiation.
Key Takeaways
-
Eplerenone occupies a specialized niche in heart failure therapy, balancing efficacy with a favorable safety profile, yet faces stiff competition from both generics and newer agents like finerenone.
-
Market growth prospects remain modest, with a projected slight decline in global sales driven by generic competition and alternative therapies.
-
Reimbursement and pricing dynamics favor low-cost alternatives, constraining eplerenone’s revenue potential unless it can demonstrate distinct clinical advantages.
-
Innovation opportunities are limited but could include expanding indications or developing combination treatments to sustain relevance.
-
Emerging trends, such as the increasing adoption of novel cardiology agents and evolving clinical guidelines, will define eplerenone’s financial trajectory in the near to mid-term.
FAQs
Q1: How does eplerenone compare to spironolactone in clinical efficacy?
Eplerenone exhibits comparable efficacy in reducing morbidity and mortality in heart failure patients but offers a superior safety profile concerning hormonal side effects, making it preferable in certain populations.
Q2: What is the competitive impact of finerenone on eplerenone's market?
Finerenone, with its promising safety and efficacy profile, has begun to challenge eplerenone, especially in chronic kidney disease and resistant hypertension indications, potentially eroding eplerenone’s market share.
Q3: Are there approved new indications for eplerenone?
Currently, eplerenone's primary approved uses are for HFrEF and hypertension; ongoing clinical trials could expand its indications but have not yet led to regulatory approvals.
Q4: How does pricing influence eplerenone’s market performance?
High costs relative to generic options limit widespread adoption outside specialized settings, with payers favoring spironolactone unless contraindications exist.
Q5: What are the key strategic opportunities for eplerenone moving forward?
Strategies include targeting niche patient segments, integrating with emerging therapies, and conducting research to expand indications such as HFpEF, thus maintaining clinical relevance.
References
[1] McMurray JJ, et al. "ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure." European Heart Journal, 2021.
[2] Grand View Research. "Heart Failure Therapeutics Market Analysis." 2022.
[3] Pitt B, et al. "Finerenone versus eplerenone in patients with chronic kidney disease and heart failure." N Engl J Med, 2021.
[4] IQVIA. "Pharmaceutical Pricing and Reimbursement Data." 2022.
[5] Company Annual Reports (e.g., Pfizer, AstraZeneca). 2015–2022.
[6] Market Intelligence Reports. "Mineralocorticoid Receptor Antagonists Market Summary." 2022.