ENTECAVIR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Entecavir, and what generic alternatives are available?
Entecavir is a drug marketed by Accord Hlthcare, Amneal Pharms, Aurobindo Pharma, Breckenridge, Brightgene, Chartwell Rx, Cipla, Conba Usa, Hetero Labs Ltd V, Prinston Inc, Rising, Sunshine, Swiss Pharm, Teva Pharms Usa, Yaopharma Co Ltd, Yung Shin Pharm, and Zydus Pharms. and is included in eighteen NDAs.
The generic ingredient in ENTECAVIR is entecavir. There are eighteen drug master file entries for this compound. Sixteen suppliers are listed for this compound. Additional details are available on the entecavir profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Entecavir
A generic version of ENTECAVIR was approved as entecavir by HETERO LABS LTD V on August 21st, 2015.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ENTECAVIR?
- What are the global sales for ENTECAVIR?
- What is Average Wholesale Price for ENTECAVIR?
Summary for ENTECAVIR
| US Patents: | 0 |
| Applicants: | 17 |
| NDAs: | 18 |
| Finished Product Suppliers / Packagers: | 15 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 249 |
| Patent Applications: | 5,196 |
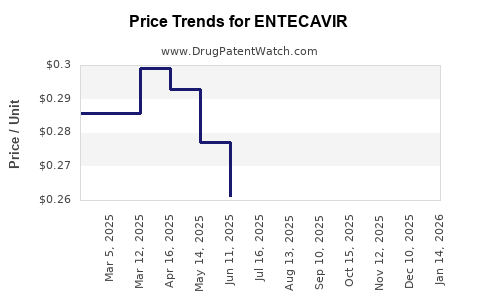
| Drug Prices: | Drug price information for ENTECAVIR |
| DailyMed Link: | ENTECAVIR at DailyMed |
Recent Clinical Trials for ENTECAVIR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University Health Network, Toronto | PHASE4 |
| Chia Tai Tianqing Pharmaceutical Group Co., Ltd. | PHASE2 |
| Sun Yat-sen University | PHASE3 |
Pharmacology for ENTECAVIR
| Drug Class | Hepatitis B Virus Nucleoside Analog Reverse Transcriptase Inhibitor |
| Mechanism of Action | Nucleoside Reverse Transcriptase Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for ENTECAVIR
US Patents and Regulatory Information for ENTECAVIR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yaopharma Co Ltd | ENTECAVIR | entecavir | TABLET;ORAL | 212201-002 | Nov 4, 2019 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Swiss Pharm | ENTECAVIR | entecavir | TABLET;ORAL | 212106-002 | Aug 10, 2020 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Yung Shin Pharm | ENTECAVIR | entecavir | TABLET;ORAL | 208195-001 | Nov 10, 2021 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| Teva Pharms Usa | ENTECAVIR | entecavir | TABLET;ORAL | 202122-001 | Aug 26, 2014 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Accord Hlthcare | ENTECAVIR | entecavir | TABLET;ORAL | 205824-001 | Aug 25, 2017 | DISCN | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | ||||
| Conba Usa | ENTECAVIR | entecavir | TABLET;ORAL | 216857-002 | Dec 23, 2024 | AB | RX | No | No | ⤷ Get Started Free | ⤷ Get Started Free | ⤷ Get Started Free | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ENTECAVIR
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Mylan Pharmaceuticals Limited | Entecavir Mylan | entecavir | EMEA/H/C/004377Entecavir Mylan is indicated for the treatment of chronic hepatitis B virus (HBV) infection in adults with:compensated liver disease and evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or fibrosis.decompensated liver disease.For both compensated and decompensated liver disease, this indication is based on clinical trial data in nucleoside naive patients with HBeAg positive and HBeAg negative HBV infection. With respect to patients with lamivudine-refractory hepatitis B.Entecavir Mylan is also indicated for the treatment of chronic HBV infection in nucleoside naive paediatric patients from 2 to | Authorised | yes | no | no | 2017-09-18 | |
| Bristol-Myers Squibb Pharma EEIG | Baraclude | entecavir | EMEA/H/C/000623Baraclude is indicated for the treatment of chronic hepatitis B virus (HBV) infection in adults with:compensated liver disease and evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or fibrosis;decompensated liver disease.For both compensated and decompensated liver disease, this indication is based on clinical trial data in nucleoside naive patients with HBeAg positive and HBeAg negative HBV infection. With respect to patients with lamivudine-refractory hepatitis B. | Authorised | no | no | no | 2006-06-26 | |
| Accord Healthcare S.L.U. | Entecavir Accord | entecavir | EMEA/H/C/004458Entecavir Accord is indicated for the treatment of chronic hepatitis B virus (HBV) infection in adults with:, , , compensated liver disease and evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active inflammation and/or fibrosis., decompensated liver disease., , , For both compensated and decompensated liver disease, this indication is based on clinical trial data in nucleoside naive patients with HBeAg positive and HBeAg negative HBV infection. With respect to patients with lamivudine-refractory hepatitis B., , Entecavir Accord is also indicated for the treatment of chronic HBV infection in nucleoside naive paediatric patients from 2 to | Authorised | yes | no | no | 2017-09-25 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
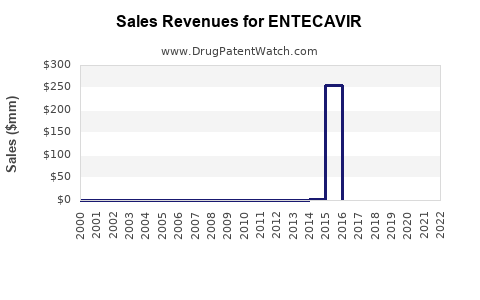
Market Dynamics and Financial Trajectory for Entacavir: A Comprehensive Analysis
More… ↓
