DULERA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Dulera, and what generic alternatives are available?
Dulera is a drug marketed by Organon Llc and is included in one NDA.
The generic ingredient in DULERA is formoterol fumarate; mometasone furoate. There are nineteen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the formoterol fumarate; mometasone furoate profile page.
Summary for DULERA
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 4 |
| Formulation / Manufacturing: | see details |
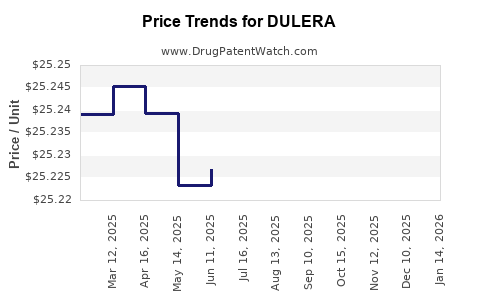
| Drug Prices: | Drug price information for DULERA |
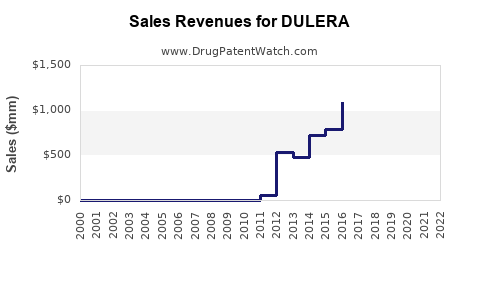
| Drug Sales Revenues: | Drug sales revenues for DULERA |
| What excipients (inactive ingredients) are in DULERA? | DULERA excipients list |
| DailyMed Link: | DULERA at DailyMed |
Recent Clinical Trials for DULERA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Sanofi | Phase 2 |
| Regeneron Pharmaceuticals | Phase 2 |
| Asthma Management Systems | Phase 4 |
Pharmacology for DULERA
| Drug Class | Corticosteroid beta2-Adrenergic Agonist |
| Mechanism of Action | Adrenergic beta2-Agonists Corticosteroid Hormone Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for DULERA
US Patents and Regulatory Information for DULERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-003 | Aug 12, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for DULERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-001 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Organon Llc | DULERA | formoterol fumarate; mometasone furoate | AEROSOL, METERED;INHALATION | 022518-002 | Jun 22, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for DULERA
See the table below for patents covering DULERA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Finland | 123580 | ⤷ Try a Trial | |
| China | 101156860 | ⤷ Try a Trial | |
| Chile | 2004001170 | ⤷ Try a Trial | |
| Denmark | 0740550 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 0051591 | ⤷ Try a Trial | |
| Denmark | 1174138 | ⤷ Try a Trial | |
| Norway | 314535 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for DULERA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435025 | 19C1040 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE GLYCOPYRROLATE (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET DE FORMOTEROL (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); NAT. REGISTRATION NO/DATE: EU/1/18/1339 20181220; FIRST REGISTRATION: - EU/1/18/1339 20181220 |
| 2435024 | 132021000000095 | Italy | ⤷ Try a Trial | PRODUCT NAME: UNA COMBINAZIONE DI FORMOTEROLO (INCLUSI SUOI SALI, ESTERI, SOLVATI O ENANTIOMERI FARMACEUTICAMENTE ACCETTABILI), GLICOPIRROLATO (INCLUSI SUOI SALI, ESTERI, SOLVATI O ENANTIOMERI FARMACEUTICAMENTE ACCETTABILI) E BUDESONIDE (INCLUSI SUOI SALI, ESTERI, SOLVATI O ENANTIOMERI FARMACEUTICAMENTE ACCETTABILI)(TRIXEO AEROSPHERE); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1498, 20201210 |
| 2435025 | 2019026 | Norway | ⤷ Try a Trial | PRODUCT NAME: KOMBINASJON AV GLYKOPYRROLAT (INKLUDERT EVENTUELLE FARMASOEYTISK AKSEPTABLE SALTER, ESTERE ELLER ENANTIOMERER DERAV) OG FORMOTEROL (INNBEFATTENDE HVILKE SOM HELST FARMASOEYTISK AKSEPTABLE SALTER, ESTERE ELLER ENANTIOMERER DERAV); REG. NO/DATE: EU/1/18/1339 20190104 |
| 2435024 | 21C1020 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI), GLYCOPYRROLATE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET BUDESONIDE (Y COMPRIS LES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); REGISTRATION NO/DATE: EU/1/20/1498 20201210 |
| 2435024 | C202130025 | Spain | ⤷ Try a Trial | PRODUCT NAME: UNA COMBINACION DE FORMOTEROL ( INCLUIDAS SUS SALES, ESTERES, SOLVATOS O ENANTIOMEROS FARMACEUTICAMENTE ACEPTABLES I, GLICOPIRROLATO ( INCLUIDAS SUS SALES, ESTERES, SOLVATOS O ENANTIOMEROS FARMACEUTICAMENTE ACEPTABLES ) Y BUDESONIDA ( INCLUIDAS SUS SALES, ESTERES, SOLVATOS O ENANTIOMEROS FARMACEUTICAMENTE ACEPTABLES ).; NATIONAL AUTHORISATION NUMBER: EU/1/20/1498; DATE OF AUTHORISATION: 20201209; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1498; DATE OF FIRST AUTHORISATION IN EEA: 20201209 |
| 2435024 | 15/2021 | Austria | ⤷ Try a Trial | PRODUCT NAME: FORMOTEROLFUMARAT-DIHYDRAT / GLYCOPYRRONIUMBROMID / BUDESONID; REGISTRATION NO/DATE: EU/1/20/1498 (MITTEILUNG) 20201210 |
| 2435024 | LUC00208 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES), DE GLYCOPYRRONIUM (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES) ET DE BUDESONIDE (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/20/1468 20201210 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


